Risc profile
- How do quinolizidine alkaloids and allergenic proteins enter the body?
- Is there a health-based guidance value?
- Is there a health risk?
- What is the quality of the available data?
- How can the health risk posed by QA and allergenic lupin proteins be reduced?
1 Subject of the assessment
Due to changes in dietary habits, the protein-rich seeds of lupin have been increasingly used as food in recent years. The health risks associated with consumption were last assessed in External Link:BfR Opinion 003/2017 of 27 March 2017. This opinion summarises the data published since then on the occurrence and toxicity of quinolizidine alkaloids, primarily taking account of lupin species whose seeds are commonly used as food. New findings on the allergenic potential of certain lupin proteins and cross-reactions with allergens from other legumes are also taken into account. This opinion does not consider health risks posed by antinutrients, mycotoxins and contaminants.
2 Result
Lupin seeds contain various toxicologically relevant substances, including quinolizidine alkaloids and allergenic proteins. In view of the current growing importance of lupin seeds, in particular the seeds of Lupinus albus, L. flavus and L. angustifolius, as food, the BfRshort forGerman Federal Institute for Risk Assessment has assessed the current state of knowledge on the possible health risks posed by quinolizidine alkaloids and allergenic proteins.
(1) Assessment of the data on health risks from exposureExposureTo glossary to quinolizidine alkaloids
To assess the health risk to humans after acute exposure, the anticholinergic effects and the influence on the electrical conduction system of the heart are currently considered to be the most sensitive toxicological endpoints, and the lowest oral effect dose of 0.16 milligrams (mgshort formilligram)/kilogram (kgshort forkilogram) body weight (BW) from human data for sparteine has been used as the toxicological reference point for a margin of exposure (MOEshort forMargin of Exposure) assessment. According to EFSA’s assessment, there are no health concerns at an MOEshort forMargin of Exposure >1. The other quinolizidine alkaloids are assumed to have a comparable effect and potency to sparteine and a group assessment with dose additivity is performed for all the compounds. The acute toxicity data available for sparteine, lupanine and 13α-hydroxylupanine, which indicate similar potency for these compounds, make this approach appear reasonable given the limited availability of data.
The available data show that acute poisoning can occur in humans after ingesting relatively large amounts of quinolizidine alkaloids. However, in connection with the consumption of foods containing lupin seeds, these have only been documented in exceptional cases to date. Due to the non-specific symptoms, however, it can be assumed that some cases go unreported.
Current data from monitoring programmes and the literature show that the levels of quinolizidine alkaloids are highest in foods in which lupin seeds make up a significant proportion, such as flours, meals and coffee substitutes. Here, levels of several hundred to thousand mgshort formilligram/kgshort forkilogram are reported in commercially available products. Tests on raw seeds, some of which are bitter varieties, show levels of over 20,000 mgshort formilligram/kgshort forkilogram total alkaloids. In other commercially available processed products that contain lupin as an ingredient, such as vegetarian spreads or milk substitutes, comparatively low total alkaloid levels of less than 50 mgshort formilligram/kgshort forkilogram have been measured. Another possible source of exposure is animal products in which quinolizidine alkaloids have been transferred from feed to animal products such as meat and milk. However, the limited available level data indicate only very low total quinolizidine alkaloid levels in such products.
(2) Evaluation of the data on allergic reactions to certain lupin proteins
With regard to allergic reactions, it can be stated in brief that relevant specialist publications have been published, in particular on prevalence in countries with a strong market presence of lupin flour products, such as France, on cross-reactivity and on the detection of various lupin allergens. In fact, about one in five people with peanut allergy have a cross-allergy to lupins, but primary sensitisation to lupins independent of peanut allergens has also been demonstrated in studies using combined, specific detection tests. Even though there are fewer reports of anaphylactic reactions in direct comparison to peanuts, this may be due to the (still) comparatively low market presence of unprocessed lupin seeds in particular. Lupin seeds are often exposed to baking and other processing processes as lupin flour in finished products, allergenic potential possibly being reduced at high temperatures. Due to these different forms of consumption, prevalence and clinical reaction data are not directly comparable, especially in individual countries. However, the severity of reaction and the symptoms of lupin allergy are very similar to the reactions in people with peanut allergy. Due to the increasing use of lupin seeds, it can be assumed that the frequency of allergic reactions, some of which can be severe, may increase.
(3) Recommendations
Due to the insufficient data available to date, there are a number of uncertainties associated with the assessment of the health risks posed by lupin seeds in food. In order to reduce these uncertainties, the following aspects should be considered:
- Collection of further data on the toxicity of quinolizidine alkaloids
- Collection of data on the consumption of foods containing lupin seeds or corresponding processed products
- Collection of data on quinolizidine alkaloid levels in food
- Measures to reduce quinolizidine alkaloid levels in food
- Methods for quantifying quinolizidine alkaloids in food
- Investigations into the allergenic potential of certain lupin proteins
- Increased information for consumers about allergenic risks
3 Rationale
3.1 Background
3.1.1 Significance of lupins to the food sector
Debittered lupin seeds have traditionally been used in Europe only for the production of snacks and consumed as such by humans (ANZFA 2001). In recent years, however, the lupin plant has steadily become more significant in the European Union (EU), particularly due to the growing demand for alternatives to animal protein sources and for domestic protein feed. In Germany, too, this development is being promoted as part of the protein crop strategy (EPS) of the Federal Ministry of Food and Agriculture (BMELshort forGerman Federal Ministry of Food and Agriculture), now the Federal Ministry of Agriculture, Food and Rural Affairs (BMLEH) (BMELshort forGerman Federal Ministry of Food and Agriculture 2020).
The Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment) has taken the growing significance of lupins in the food sector as an opportunity to evaluate new data on possible health risks associated with the consumption of lupin seeds and foods made from them that has become available since the publication of BfRshort forGerman Federal Institute for Risk Assessment Opinion 003/2017 in 2017. The focus has been on quinolizidine alkaloids and allergenic lupin proteins. As part of a literature review focusing in particular on the period from 2017 to 2022, data on toxicokinetics and toxicodynamics, animal studies, human data on adverse health effects and current data on the occurrence of quinolizidine alkaloids have been evaluated. In addition, data on allergic reactions caused by the consumption of foods containing lupin seeds have been taken into account.
3.1.2 Quinolizidine alkaloids in lupin seeds and foods containing lupin seeds
Lupin varieties that produce seeds with a low alkaloid content and have been developed through targeted breeding are referred to as “sweet lupins”, while those whose seeds taste bitter due to higher alkaloid levels are referred to as “bitter lupins”. However, there are no binding food law requirements in the EU specifying the alkaloid content at which a lupin species or variety is to be assigned to a particular category. Varieties with a total alkaloid content in the grain of 500 mgshort formilligram/kgshort forkilogram or less (≤0.05 % dry matter) are often referred to as sweet lupins, while varieties with a total alkaloid content of 10,000 mgshort formilligram/kgshort forkilogram or more (≥1 % dry matter) are referred to as bitter lupins (Pilegaard & Gry 2008). Other authors specify a range of 100–800 mgshort formilligram/kgshort forkilogram for sweet lupins (Gessner & Orzechowski 1974). According to Delegated Regulation (EU) 2022/1104 amending Regulation (EU) No 68/2013 on the catalogue of feed materials, sweet lupins are defined as seeds of Lupinus spp. with a maximum content of 5 % bitter seeds. The Australian New Zealand Food Authority (ANZFA) assumes average alkaloid contents of 130–150 mgshort formilligram/kgshort forkilogram for sweet lupin seeds (ANZFA 2001). However, there are also semi-sweet and semi-bitter varieties (Boschin et al. 2008).
In 1996, the Advisory Committee on Novel Food and Processes (ACNFP) in the United Kingdom published a health assessment of the seeds of L. angustifolius (FSA 1996). The committee concluded that the use of L. angustifolius seeds for food production is safe, provided that the total alkaloid content in the seeds or lupin products does not exceed 200 mgshort formilligram/kgshort forkilogram. This corresponds to the maximum permitted concentration (MPC) already permitted in Australia (ANZFA 2001).
In its opinion, the Australia New Zealand Food Authority (ANZFA) wrote that the only data available on chronic toxicity comes from reports on the traditional use of lupin seeds in Europe. It was deduced from the data that a daily intake of 0.35 mgshort formilligram/kgshort forkilogram of lupin alkaloids is tolerated by adults without adverse effects. Applying a safety factor of 10 to account for uncertainties in the data and, in particular, to account for likely individual variations, ANZFA derived a provisional tolerable daily intake (PTDI) for lupin alkaloids for adults of 0.035 mgshort formilligram/kgshort forkilogram body weight (BW). The average alkaloid content of lupin seeds on the Australian market is 130–150 mgshort formilligram/kgshort forkilogram (ANZFA 2001).
In the past, individual EU Member States had regulations governing the use of lupins as food. In France, for example, the use of up to 10 % lupin flour was accepted, provided that the flour came from the seeds of a low-alkaloid variety of L. albus and the alkaloid content did not exceed 200 mgshort formilligram/kgshort forkilogram (Direction générale de la santé & Bureau VS 3 1998).
There are currently no legally binding maximum levels for quinolizidine alkaloids in food in the EU.
3.1.3 Allergenic proteins in lupins
Certain proteins in lupins, which are largely heat-stable, have allergenic potential and can cause cross-reactions with the allergens in other legumes. For these reasons, “lupin” and “lupin products” have been included in the group of allergens subject to labelling requirements, which must be indicated as ingredients in food under all circumstances on the labelling of food. By definition, the labelling requirement also applies if lupin products (flour or protein/fibre concentrates) are added to food in only small quantities due to their emulsifying properties.
In Switzerland and other countries such as Australia, New Zealand, Morocco, Turkey and Ukraine, the potential presence of lupin allergens must also be declared on products.
3.2 Risk assessment
3.2.1 Hazard identification
The genus Lupinus spp. belongs to the legume family (Fabaceae or Leguminosae). Up to 500 different lupin species are described in the literature, with the majority of these species, the “New World” species, only being found in North or South America. In Europe and North Africa, on the other hand, there are only twelve lupin species, the “Old World” species (Wink et al. 1995; Boschin & Resta 2013).
However, only four lupin species are cultivated on a relatively large scale worldwide as food and feed: L. albus (white lupin), L. angustifolius (blue lupin), L. luteus (yellow lupin) and L. mutabilis Sweet (Andean lupin). L. mutabilis, however, is less widely cultivated with Andean lupin not being cultivated for commercial purposes in Europe (EFSAshort forEuropean Food Safety Authority 2019).
Due to their high protein content and favourable amino acid composition, lupin seeds serve as a plant-based protein source for food and feed production. In Europe, they are traditionally consumed as a snack (Gresta et al. 2010; Boschin & Resta 2013; Carvajal-Larenas et al. 2016; Magalhães et al. 2017). In addition, lupin seeds are now mainly processed into flour or meal and used as an ingredient in various foods, e.g. in numerous meat, milk, egg and soy substitutes as well as various spreads. Other food categories in which lupin seeds or flour made from them are used include bread products, dairy products such as yoghurts and ice creams, sauces, confectionery and baked goods, pasta, protein powder, plant-based drinks and coffee substitutes.
3.2.1.1 Quinolizidine alkaloids in lupin seeds
The seeds, as well as the other parts of the lupin plant, contain quinolizidine alkaloids, which are toxicologically relevant, bitter-tasting secondary plant compounds. Levels can vary greatly depending on the botanical and geographical origin of the plant, as well as the soil composition and climatic conditions (Khan et al. 2015; Wink 2019).
Quinolizidine alkaloids are most commonly found in plants of the Fabaceae family, particularly in the lupin genus. The more than 170 representatives described in the literature consist of a quinolizidine backbone and can be classified in particular by the number of ring structures. In addition to bicyclic (e.g. lupinine) and tricyclic (e.g. angustifoline) quinolizidine alkaloids, tetracyclic quinolizidine alkaloids are also known, which can be further divided into sparteine-like (e.g. sparteine) and matrine-like (e.g. matrine) quinolizidine alkaloids. In addition, there are a number of quinolizidine alkaloids that cannot be assigned to any of these groups due to their differing structure (Griffiths et al. 2021; Mancinotti et al. 2022).
3.2.1.1.1 Main alkaloids of lupin species commonly cultivated for food production
Quinolizidine alkaloids are synthesised in the green organs of lupin plants, transported via the phloem and stored in all organs of the plant. The genes involved in biosynthesis have been found to be most strongly expressed in the epidermis. A particularly high concentration of quinolizidine alkaloids is found in the seeds of the plants (Frick et al. 2023; Rodes-Bachs & Van der Fels-Klerx 2023). The distribution of quinolizidine alkaloids in different parts of the plant is selective, such that the leaves of lupin plants have a much more diverse quinolizidine alkaloid profile than the seeds (Wink et al. 1995). It is not yet known whether quinolizidine alkaloids can also be synthesised in the seeds.
Despite numerous studies, the biosynthesis of quinolizidine alkaloids has only been partially elucidated. Almost all quinolizidine alkaloids are formed from the amino acid L-lysine. The enzyme lysine decarboxylase converts L-lysine into cadaverine. A cascade of reactions produces the bicyclic (-)-lupinine and, via the diiminium cation as a further intermediate product, the tetracyclic quinolizidine alkaloids such as (-)-sparteine and (+)-lupanine (Golebiewski & Spenser 1988). Other structurally different quinolizidine alkaloids can be formed as a result of further reactions such as hydroxylations or esterifications (Bunsupa et al. 2012).
The various lupin species and varieties differ both in the quinolizidine alkaloid content of the lupin seeds and in the profile of quinolizidine alkaloids present. The following list shows which quinolizidine alkaloids make up the majority of the total quinolizidine alkaloid content in the seeds of the lupin species most commonly cultivated for food production worldwide.
- Lupinus albus
Lupanine is the main alkaloid (up to 97 % of the total content of the quinolizidine alkaloids examined) in both the quinolizidine alkaloid-rich “bitter” varieties and the quinolizidine alkaloid-poor “sweet” varieties. Other main alkaloids are albine (up to 26 %), 13α-hydroxy-(OH)-lupanine (up to 24 %) and multiflorine (up to 11 %). The primary secondary alkaloids are 13α-angeloyloxylupanine, angustifoline, isolupanine and, in some cases, sparteine. While the total content of the quinolizidine alkaloids examined in the seeds of “bitter” lupin varieties can be as high as 52,380 mgshort formilligram/kgshort forkilogram, there are sweet lupin varieties of albus whose total content in the seeds is only 40 mgshort formilligram/kgshort forkilogram (Boschin et al. 2008; Erbas 2010; Gresta et al. 2010; Magalhães et al. 2017; Romeo et al. 2018). - Lupinus luteus
Depending on variety, the main alkaloids in the seeds of luteus are sparteine (up to 97 % of the total content of the quinolizidine alkaloids examined) and lupinine (up to 46 %). 13α-OH-lupanine and lupanine are the most common secondary alkaloids. The total alkaloid content in the seeds of sweet lupin varieties can be less than 10 mgshort formilligram/kgshort forkilogram, while seeds of bitter L. luteus varieties have alkaloid contents of over 10,000 mgshort formilligram/kgshort forkilogram (Gresta et al. 2010; Magalhães et al. 2017; Romeo et al. 2018). - Lupinus angustifolius
Depending on variety, lupanine is the main alkaloid in the seeds of angustifolius, accounting for up to 82 % of the total content of the quinolizidine alkaloids examined. Other main alkaloids are 13α-OH-lupanine (up to 47 %), angustifoline (up to 31 %) and isolupanine (up to 14 %). Minor alkaloids include sparteine, tetrahydrorhombifoline and multiflorine. Depending on variety, the total alkaloid content in the seeds of L. angustifolius can range from 15 to 25,000 mgshort formilligram/kgshort forkilogram (Christiansen et al. 1997; de Cortes Sánchez et al. 2005; Resta et al. 2008; Gresta et al. 2010; Chilomer et al. 2011; Magalhães et al. 2017). - Lupinus mutabilis
The main alkaloids in the seeds of mutabilis varieties are lupanine (up to 88 % of the total content of the quinolizidine alkaloids examined), sparteine (up to 23 %) and 13α-OH-lupanine (up to 15 %). The primary secondary alkaloids are tetrahydrorhombifoline and 13α-angeloyloxylupanine. Quinolizidine alkaloid-rich “bitter” varieties of L. mutabilis have a high total alkaloid content of up to 60,000 mgshort formilligram/kgshort forkilogram dry matter (DM) (Hatzold et al. 1983; Cortés-Avendaño et al. 2020).
Table 1 provides an overview of the main alkaloids found in lupin species commonly cultivated for food production. A detailed table listing the quinolizidine alkaloids found in different lupin species and varieties and their proportions of the total alkaloid content can be found in External Link:EFSA’s 2019 opinion (EFSAshort forEuropean Food Safety Authority 2019).
Table 1: Main alkaloids in lupin species commonly cultivated for food productiona.
| Lupanine | 13α-OH-Lupanine | Albine | Sparteine | Lupinine | Angustifoline | |
| L. albus | up to 97 % | up to 24 % | up to 26 % | - | - | - |
| L. luteus | - | - | - | up to 97 % | up to 46 % | - |
| L. angustifolius | up to 82 % | up to 47 % | - | - | - | up to 31 % |
| L. mutabilis | up to 88 % | - | - | up to 23 % | - | - |
a Only the main alkaloids, which can account for at least 20 % of the total, are listed.
Currently, the analysis of the group of quinolizidine alkaloids, which comprises more than 170 compounds, is limited to only a few representatives, meaning that only some of the quinolizidine alkaloids found in plants are recorded. Due to the limited availability of data, it is not yet possible to assess the health relevance of these quinolizidine alkaloids which are not analytically detectable. Furthermore, the content and alkaloid profile in the plants are subject to considerable variation. For example, climatic factors such as temperature, drought and light, but also soil conditions and the cultivation system (organic/conventional) can influence alkaloid content, meaning that differences are also possible within the same varieties (Rodes-Bachs & Fels-Klerx 2023). Therefore, the percentages given should be used for guidance.
3.2.1.1.2 Influence of environmental conditions on quinolizidine alkaloid content
The quinolizidine alkaloid content in lupin seeds of one and the same genotype can be influenced by a variety of environmental factors. The most important factors are light, dryness and ambient temperature (Frick et al. 2017; Tirdilova et al. 2022).
The synthesis of quinolizidine alkaloids takes place in the chloroplasts and is therefore dependent on the time of day and light (Boschin & Resta 2013). The enzymes involved in the synthesis of quinolizidine alkaloids are light-sensitive; depending on the brightness, they are either more active or inhibited. In addition, the pH value of the chloroplast stroma changes from pH 7 in the dark to pH 8 in the light. Since lysine decarboxylase and other relevant enzymes have an optimum pH of 8 and are significantly less active at pH 7, light in turn promotes quinolizidine alkaloid biosynthesis. In addition, lysine decarboxylase is activated by reduced thioredoxin (Wink & Hartmann 1981).
It is generally assumed that drought increases alkaloid levels in lupin plants; however, the stage of plant development at which the drought occurs is also significant. Alkaloid content changes throughout the growth period of the lupin plant, with alkaloids accumulating in the seeds and roots towards the end of the life cycle (Hondelmann 1984).
Increased rainfall during fruit formation and ripening, for example, led to a lower average alkaloid content in the seeds of L. angustifolius (263.6 mgshort formilligram/100 g DM vs. 501.7 mgshort formilligram/100 g (Vishnyakova et al. 2023)).
Christiansen et al. investigated the influence of environmental factors on the quinolizidine alkaloid content of different L. angustifolius varieties during the different growth phases of the plant. During the vegetative phase of the lupin plant, drought increases the alkaloid content in both varieties with high and low levels. During the flowering period, the alkaloid content decreased under the influence of drought, while it increased again during the fruit ripening phase (Christiansen et al. 1997). High air temperatures during seed ripening also cause the alkaloid content in the seeds of L. angustifolius to increase (Jansen et al. 2009).
In addition, the proportionate composition of the alkaloid profile can also change under the influence of environmental factors. For example, under the influence of drought during the seed maturation phase, the proportion of sparteine in the total alkaloid content decreased, while the proportion of isoangustifoline increased (Christiansen et al. 1997). However, this always depends on the variety being studied. Some L. angustifolius varieties are only slightly affected by drought or temperature stress, while others are not affected by climatic conditions at all and have a constant quinolizidine alkaloid content regardless of these conditions (Rodes-Bachs & Van der Fels-Klerx 2023).
In contrast, cultivation in regions with a subcontinental climate with lower average temperatures and increased rainfall led to significantly higher quinolizidine alkaloid contents in the seeds of all L. albus varieties studied compared to cultivation in locations with a Mediterranean climate (Boschin et al. 2008).
Annicchiarico et al. confirmed these observations by comparing L. albus varieties from two locations, one with a subcontinental climate and the other with a Mediterranean climate. Winter cold stress, a rainy spring and subsequent drought stress led to higher yields across all varieties, but also to significantly higher quinolizidine alkaloid contents (Annicchiarico et al. 2014).
3.2.1.1.3 Industrial debittering of lupin seeds
The literature describes research results on industrial debittering processes for lupin seeds (Haddad et al. 2006; Carvajal-Larenas et al. 2013; Ertas & Bilgicli 2014), which are based in particular on the good water solubility of quinolizidine alkaloids in their salt form and in some cases also include fermentation processes (Jiménez-Martínez et al. 2007; Ortega-David & Rodriguez-Stouvenel 2013).
Carvajal-Larenas et al. distinguished between biological processes, chemical extractions and aqueous debittering. Biological methods are mostly based on fermentation by bacteria or fungi, but also include rinsing and boiling steps. In chemical extraction with a base, the alkaloids, some of which occur as salts, are converted into free bases. In a following step, the free alkaloids can then be removed by extraction with organic solvents (Ortiz & Mukherjee 1982). It should be noted that residues of the solvents must also be removed in several rinsing processes. In aqueous debittering processes, several soaking, rinsing and boiling steps follow one another in order to effectively reduce the alkaloid content (Carvajal-Larenas et al. 2016).
The various industrial debittering processes differ in their effectiveness in reducing alkaloid content. Biological methods, mainly based on bacterial fermentation, have been shown to reduce the alkaloid content of L. albus by 40–55 % (Camacho et al. 1991; Santana & Empis 2001) and by 91 % in L. mutabilis seeds through fermentation with the fungus Rhizopus oligosporus after prior soaking and boiling (Jiménez-Martínez et al. 2007). Various aqueous methods proved to be most effective, removing 80 % (Villacrés et al. 2020) or even over 99 % of the alkaloids from L. mutabilis, depending on the process (Torres Tello et al. 1980; Aguilera et al. 1983; Cortés-Avendaño et al. 2020).
3.2.1.1.4 Non-industrial debittering methods
Methods have also been described for household debittering, most of which are based on a combination of boiling and soaking for a number of days with repeated water changes (Bleitgen et al. 1979; Smith 1987; Fudiyansyah et al. 1995; Lowen et al. 1995; Pilegaard & Gry 2008; Ertas & Bilgicli 2014). A typical debittering method described by various authors is based on instructions provided by Lowen et al.: first, six parts of cold water are added to the lupin seeds for each part of seeds. After soaking for 24 hours, the water is poured off, the lupin seeds are rinsed and boiled with the same volume of water as described above for 7–10 minutes. After rinsing again, all steps are repeated for 5–7 days until the lupin seeds no longer taste bitter (Smith 1987; Lowen et al. 1995). Smith analysed the decrease in alkaloids during the individual debittering steps and found that alkaloids were still being transferred to the soaking water on the sixth day of soaking (Smith 1987). Debittering can be further enhanced by a pH value of 2.2–2.4, as the water solubility of alkaloids increases at lower pH values. Citric acid can be added for this purpose. The addition of table salt also promotes the leaching of alkaloids (FiBL 2024).
Bleitgen et al. conducted experiments on the debittering of seeds of L. mutabilis var. H-1 and L. albus var. Astra using sensory testing (Bleitgen et al. 1979). They found that the swelling speed and swelling capacity of the lupin seeds are important for debittering and that the boiling process increases the leaching of alkaloids from the seeds. Based on their findings, the authors recommended boiling the whole lupin seeds for half an hour and soaking them in running water for three days to remove the bitterness at home. The results also showed that the bitter taste of lupin alkaloids in water could still be detected by taste in the ppm range in the case of sparteine. The lupin alkaloids differed greatly in their degree of bitterness. This decreased from D-lupanine perchlorate, lupinine and isolupanine to 13α-OH-lupanine. The swelling capacity of lupin seeds depended on the lupin species. The swelling rate was lower for seeds of L. albus than for seeds of L. mutabilis.
Overall, however, it can be said that there are no systematic and validated studies on the quality of culinary debittering methods. In addition, the aqueous debittering process also washes out many water-soluble nutrients such as vitamins, minerals and flavonoids (Villacrés et al. 2020).
It is assumed that the success of debittering measures depends on various parameters and also on the variable initial content of lupin alkaloids in the seeds. Cases of poisoning have repeatedly been attributed to insufficient debittering of bitter lupin seeds using culinary techniques (see section 3.2.2.1.6). This illustrates that the debittering of lupin seeds by consumers is a critical step on which the safety of the food depends. Other than a sensory test to determine whether the seeds still taste bitter or not, there is also currently no way for consumers to test the remaining alkaloid content.
Given the current insufficient level of knowledge, the BfRshort forGerman Federal Institute for Risk Assessment is therefore unable to issue any general recommendations on methods for removing the bitterness from bitter lupin seeds in the kitchen.
3.2.1.1.5 Analytical determination of quinolizidine alkaloid content
Initially, titration and thin-layer chromatography methods were mainly used to detect quinolizidine alkaloids (Ruiz Jr. 1977; Ruiz Jr. et al. 1977; Karlsson & Peter 1978; Muzquiz et al. 1994; EFSAshort forEuropean Food Safety Authority 2019). Since the 1980s, methods based on gas chromatography (GC) have been the main methods used for the identification and (semi)quantification of quinolizidine alkaloids in various matrices (BfRshort forGerman Federal Institute for Risk Assessment 2017; EFSAshort forEuropean Food Safety Authority 2019). Over the past five years, the focus on the quantitative determination of quinolizidine alkaloids has shifted significantly towards methods based on liquid chromatography-mass spectrometry (LC-MS).
3.2.1.1.6 GC-MS-based methods
Today’s GC-based methods for the determination of quinolizidine alkaloids are very similar to the methods developed in the 1980s. Sample preparation usually involves acid extraction, alkalisation of the extracts and subsequent liquid-liquid or solid-phase extraction (Wink et al. 1995; Boschin et al. 2008; Kamel et al. 2015). GC-FID (flame ionisation detector), GC-NPD (nitrogen-phosphorus detector) and GC-MS(EI) methods are primarily used. Chromatographic separation is performed using nonpolar columns (BfRshort forGerman Federal Institute for Risk Assessment 2017; EFSAshort forEuropean Food Safety Authority 2019). With the GC-MS methods commonly used today, the identification of quinolizidine alkaloids is primarily based on the obtained mass spectra and comparison with spectrometric databases (Wink et al. 1995; Chludil et al. 2009). Due to the lack of reference standards, quinolizidine alkaloids are usually quantified by comparing relative peak areas to available standards such as sparteine and lupanine, which means that the obtained contents of the other compounds may be subject to a high degree of uncertainty (Boschin et al. 2008; Resta et al. 2008; Romeo et al. 2018; Cely-Veloza et al. 2022). In some publications, quantification is carried out using the individual compounds. In earlier work, the relevant quinolizidine alkaloids were first isolated from plant material and purified for subsequent use as standards (Priddis 1983; Reinhard et al. 2006). In current publications, commercially available reference standards are used (Cortés-Avendaño et al. 2020; Lee et al. 2020). In most cases, there is no need for derivatisation of the quinolizidine alkaloids. However, derivatisation can be helpful in order to achieve higher sensitivity or to avoid matrix effects in complex matrices such as food (Reinhard et al. 2006). The detection and quantification limits reported vary depending on the method, analyte and matrix. For lupin flour, for example, Reinhard et al. reported detection limits between 0.3 and 6 mgshort formilligram/kgshort forkilogram (Reinhard et al. 2006).
3.2.1.1.7 LC-MS-based methods
Until the BfRshort forGerman Federal Institute for Risk Assessment issued its opinion on the presence of alkaloids in lupin seeds in 2017 (BfRshort forGerman Federal Institute for Risk Assessment 2017) and the EFSAshort forEuropean Food Safety Authority issued its opinion on quinolizidine alkaloids in food and feed in 2019 (EFSAshort forEuropean Food Safety Authority 2019), only isolated LC-MS-based methods for the detection of quinolizidine alkaloids in various matrices (e.g. blood (Green et al. 2015), plant parts (Otterbach et al. 2019)) had been described in the literature (Mol et al. 2011; Przybył & Kubicki 2011; Carlier et al. 2015; Green et al. 2015; Lee et al. 2019; Otterbach et al. 2019).
Since then, LC-MS-based methods, especially for the quantification of quinolizidine alkaloids in lupin seeds and foods containing lupin seeds, have been increasingly developed, validated to varying degrees and applied.
In 2020, Hwang et al. developed, optimised and validated a UHPLC-MS/MS-based method for the detection of five commercially available quinolizidine alkaloids of sufficient purity (lupanine, 13α-OH-lupanine, angustifoline, sparteine and lupinine) in seeds of the narrow-leaved lupin (L. angustifolius) and in foods produced from them (e.g. pasta, milk substitutes, biscuits) (Hwang et al. 2020). Among other things, the mean recovery rates were determined. These range between 89 and 108 % and were determined in spiking experiments with three spiking levels (25 mgshort formilligram/kgshort forkilogram, 500 mgshort formilligram/kgshort forkilogram and 2,000 mgshort formilligram/kgshort forkilogram).
In April 2022, the European Reference Laboratory for Mycotoxins and Phytotoxins in Food and Feed (EURL-MP, Wageningen Food Safety Research (WFSR)) published the protocol for an LC-MS/MS method for the determination of fifteen quinolizidine alkaloids (albine, anagyrine, angustifoline, trans-13α-cinnamoyloxylupanine, cytisine, epilupinine, gramine, 13α-OH-lupanine, isolupanine, lupanine, lupinine, methylcytisine, multiflorine, sparteine, thermopsine) in lupin seeds, cereal-based foods, meat and milk substitutes, milk and compound feed on its website (EU Reference Laboratory for mycotoxins & plant toxins in food and feed 2022). The individual laboratory validation data have not been published.
In 2024, WFSR and several Israeli institutes published the results of their investigation into the quinolizidine alkaloid content of Israeli wild lupin seeds (L. pilosus and L. palaestinus) (Namdar et al. 2024) using the LC-MS/MS method for fifteen commercially available quinolizidine alkaloids as analytical standards. In addition, a GC-MS method known from the literature was used to identify and semi-quantify several quinolizidine alkaloids for which no standards are commercially available. The results of the single laboratory validation collected as part of the study were also presented. In spiking experiments with three levels (1 mgshort formilligram/kgshort forkilogram, 5 mgshort formilligram/kgshort forkilogram and 25 mgshort formilligram/kgshort forkilogram), recoveries between 80 % and 155 % (with the exception of trans-13α-cinnamoyloxylupanine at 45 %–55 %) and relative standard deviations (n = 6) between 2 % and 14 % were determined for all analytes investigated. The limit of quantification is 1 mgshort formilligram/kgshort forkilogram.
In September 2022, the BfRshort forGerman Federal Institute for Risk Assessment published the results of a study on the transfer of quinolizidine alkaloids from narrow-leaved lupin (L. angustifolius) into milk from dairy cows (Engel et al. 2022). In this context, the BfRshort forGerman Federal Institute for Risk Assessment reports two in-house validated LC-MS/MS methods for the determination of nine quinolizidine alkaloids (anagyrine, angustifoline, cytisine, 13α-OH-lupanine, isolupanine, lupanine, lupinine, multiflorine and sparteine) in lupin seeds and cow’s milk. For both methods, recovery rates between 80 and 110 % and intra-laboratory repeatability and reproducibility of less than 10 % were reported. The limits of detection and quantification for the method for determining the nine quinolizidine alkaloids in lupin seeds are less than 1 mgshort formilligram/kgshort forkilogram and less than 2 mgshort formilligram/kgshort forkilogram, respectively, and for the method for determining quinolizidine alkaloids in milk, less than 0.001 mgshort formilligram/kgshort forkilogram and less than 0.002 mgshort formilligram/kgshort forkilogram, respectively.
In 2023, Eugelio et al. published the results of the development and validation of an LC-MS/MS-based method for the identification and quantification of thirteen commercially available quinolizidine alkaloids (N-formylcytisine, cytisine, 13α-OH-lupanine, N-methylcytisine, lupinine, albine, angustifoline, multiflorine, thermopsine, lupanine, gramine and sparteine) in lupin seeds (Eugelio et al. 2023). The method provides recovery rates between approximately 60 and 110 % and limits of detection between 0.001 and 0.025 mgshort formilligram/kgshort forkilogram. Lupin seed samples from L. albus were analysed using the validated method.
Keuth et al. also reported in 2023 on the investigation of a total of 30 lupin seed-based food products from retailers in North Rhine-Westphalia between 2019 and 2021 (Keuth et al. 2023). The food samples (e.g. bitter lupin seeds, lupin flour, wholemeal bread, lupin-based milk substitutes and coffee substitutes) were tested for five commercially available quinolizidine alkaloids (13α-OH-lupanine, lupanine, lupinine, angustifoline and sparteine) using LC-MS/MS. Detection and quantification limits of 0.01 to 0.1 mgshort formilligram/kgshort forkilogram and 0.02 to 0.15 mgshort formilligram/kgshort forkilogram, respectively, were determined for the method used.
In 2023, Schryvers et al. investigated the effect of different processing methods on the quinolizidine alkaloid content in lupin seeds (L. albus) and in lupin seed-based foods (Schryvers et al. 2023). In these studies, a UHPLC-MS/MS method based on the methods of Horna (Vanerková et al. 2014) and Hwang (Hwang et al. 2020) was developed and validated for the quantification of five quinolizidine alkaloids (sparteine, lupanine, lupinine, 13α-OH-lupanine and angustifoline) in lupin seeds and matrices with a high fat content (biscuits). The limit of quantification of this method is 0.05 mgshort formilligram/kgshort forkilogram for sparteine and angustifoline and 0.1 mgshort formilligram/kgshort forkilogram for lupanine, lupinine and 13α-OH-lupanine. The recovery rates determined for the method range between 76 and 110 % for three investigated spiking levels per analyte (spiking levels between 50 µgshort formicrogram/kgshort forkilogram and 10,000 µgshort formicrogram/kgshort forkilogram). In addition, a screening method using UHPLC-HR-MS was applied to identify other relevant quinolizidine alkaloids for which no standard substances are available in lupin seeds and foods containing lupin seeds.
In a subsequent study, Schryvers et al. investigated the transfer of quinolizidine alkaloids into animal-based foods (veal and calf’s liver) (Schryvers et al. 2024a). For this purpose, the UHPLC-MS/MS method was extended and validated for a total of seven analytes (lupanine, 13α-OH-lupanine, lupinine, sparteine, angustifoline, multiflorine and albine) and the relevant matrices (lupin seeds, mixed feed containing lupin seeds, calf’s liver, veal). The limits of quantification determined are 0.01 mgshort formilligram/kgshort forkilogram for the majority of the analyte-matrix combinations investigated and 0.05 mgshort formilligram/kgshort forkilogram at most. In spiking experiments with three levels (10 or 50 µgshort formicrogram/kgshort forkilogram, 200 µgshort formicrogram/kgshort forkilogram and 1,000 µgshort formicrogram/kgshort forkilogram), recovery rates between 74 % and 114 % were determined (with the exception of angustifoline in veal, which was 64 %–69 %). Intra-laboratory repeatability and reproducibility was below 20 % for all analyte-matrix combinations examined.
In all of the methods described above, the extraction of quinolizidine alkaloids is based on solid-liquid or liquid-liquid extraction using a mixture of water and an organic solvent (e.g. methanol, acetonitrile) and, if necessary, formic acid, followed by centrifugation, dilution and, if necessary, filtration. In the case of the method presented by the BfRshort forGerman Federal Institute for Risk Assessment, sample preparation additionally includes protein precipitation at -80°Celsius (C) and, for some matrices, degreasing with n-hexane (Engel et al. 2022). The method described by Eugelio et al. additionally includes solid-phase extraction (SPE) (Eugelio et al. 2023). For calf’s liver and veal, Schryvers et al. use dispersive solid-phase extraction (dSPE) (Schryvers et al. 2024a).
The LC-MS/MS method developed and validated in 2023 by Khedr et al. for the determination of five quinolizidine alkaloids (angustifoline, isolupanine, 13α-OH-lupanine, lupanine and sparteine) in lupin seeds, on the other hand, uses a modified QuEChERS sample preparation method (Khedr et al. 2023). For this method, a limit of quantification of 0.01 mgshort formilligram/kgshort forkilogram and recovery rates between 72 and 109 % were determined for all the quinolizidine alkaloids investigated. The newly developed method was used to examine the quinolizidine alkaloid profile of different varieties of five lupin species (L. angustifolius, L. cosentinii, L. albus, L. luteus, L. mutabilis).
In September 2022, the Austrian Agency for Health and Food Safety (AGES) presented an LC-MS/MS multi-method for twelve quinolizidine alkaloids in dry and liquid foods at the 11th Symposium on “Recent Advances in Food Analysis” and published an excerpt in the abstract volume from the symposium (Czerwenka & Dorn 2022). Details about the analytes and matrices examined and on the validation were not published in the excerpt.
3.2.1.1.8 Reference substances, certified reference materials and suitability tests
An important but still limiting factor in the quantification of quinolizidine alkaloids is the availability of commercially available reference standards of sufficient purity. This limitation has already been described by the BfRshort forGerman Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment 2017) and the EFSAshort forEuropean Food Safety Authority (EFSAshort forEuropean Food Safety Authority 2019) and persists to this day. A slowly increasing number of available standard substances is reflected in a growing range of reliably quantifiable analytes in recent publications. Isotope-labelled standards are still not commercially available. However, suitability tests for the determination of quinolizidine alkaloids in food and, in this context, reference materials have been commercially available for the first time since 2023.
3.2.1.2 Allergenic proteins in lupins
In addition to quinolizidine alkaloids, certain largely heat-stable allergenic proteins in lupins are toxicologically relevant. They can cause cross-reactions with the allergens of other legumes and lead to cross-allergies. Isolated sensitisation and allergies to lupin protein have also been described.
3.2.2 Hazard characterisation
3.2.2.1 Data on the toxicity of quinolizidine alkaloids
The following is a summary of data relevant to the assessment of the toxicity of the seeds of L. albus, L. angustifolius, L. luteus and L. mutabilis and the quinolizidine alkaloids they contain after oral exposure.
3.2.2.1.1 Toxicokinetics
Very little data is available on the toxicokinetics of quinolizidine alkaloids. The literature search yielded few new findings. The most important information on toxicokinetics is summarised below. This information is primarily taken from previous opinions from the BfRshort forGerman Federal Institute for Risk Assessment in 2017 and the EFSAshort forEuropean Food Safety Authority in 2019 (BfRshort forGerman Federal Institute for Risk Assessment 2017; EFSAshort forEuropean Food Safety Authority 2019).
It is known that sparteine is absorbed from the gastrointestinal tract at a rate of 70 % in humans after oral administration, with maximum plasma concentrations being reached after 45 minutes. After intravenous administration of sparteine sulfate, 34 % of the substance is excreted unchanged in the urine within 24 hours. Approximately 50 % of sparteine is bound to plasma proteins. Sparteine is metabolised by the cytochrome Pshort forphosphorus-450 isoenzyme CYP2D6 which, however, 5-10 % of the population do not possess as a functional enzyme due to genetic polymorphism. Such poor metabolisers (PM), as they are known, have both higher sparteine plasma levels and a longer plasma half-life than the normal population after exposure and excrete more than 95 % of the orally administered dose as unchanged sparteine in their urine. These circumstances make non-metabolisers more sensitive to undesirable sparteine effects than individuals with functional CYP2D6 metabolism (Schomerus et al. 1978; Eichelbaum et al. 1979; Thies 1986; Blaschek et al. 2006; Aktories et al. 2009).
Wittenburg and Nehring administered lupanine hydrochloride to rats in their feed. Between 70 and 80 % of the lupanine ingested was excreted, with 50–70 % excreted in the urine and only 10 to 14 % eliminated in the faeces. Approximately half of the lupanine ingested was excreted as hydroxylated lupanine and approximately the same proportion was excreted unchanged in the urine or faeces (Wittenburg & Nehring 1965). In another study on rats, after administration of 10 mgshort formilligram lupanine, half-lives of 6.2 ± 0.5 h (EM) and 6.5 ± 0.9 h (PM) were observed for “normal” metabolisers (“extensive metabolisers” (EM)) and four non-metabolisers in relation to CYP2D6 (Petterson et al. 1994).
3.2.2.1.2 Toxicodynamics
Several studies have demonstrated in vitro interaction of lupin alkaloids with isolated nicotinic and muscarinic acetylcholine receptors (Yovo et al. 1984; Schmeller et al. 1994).
Further studies showed that sparteine blocks sodium channels and reduces the potassium permeability of nerve and pancreatic cells. In addition, sparteine and lupanine (300 μM each) inhibit the sodium and potassium channels of isolated Xenopus laevis frog muscle cells (Blaschek et al. 2023).
Lupin alkaloids had a uterine-contracting effect ex vivo. In isolated rabbit uterus, lupinine is only 1/5, and lupanine dihydrochloride only 1/15 as potent as sparteine disulfate (Ligon 1941; Gessner & Orzechowski 1974). Lupin alkaloids have an antiarrhythmic effect on the isolated heart by eliminating atrial and ventricular flutter through slowing the conduction of electrical impulses. The antiarrhythmic effect decreases from sparteine to lupanine to 13α-OH-lupanine (Czarnecka et al. 1967; Raschack 1974).
While most experimental data on isolated quinolizidine alkaloids are only available for sparteine and lupanine, little is known about the toxicokinetic and toxicodynamic behaviour of other potentially relevant quinolizidine alkaloids such as albine or angustifoline.
3.2.2.1.3 Acute, subacute, subchronic and chronic toxicity
Only a few studies are available on acute, subacute, subchronic and chronic toxicity, which are already listed in detail in BfR’s 2017 opinion and the EFSA’s 2019 opinion (BfRshort forGerman Federal Institute for Risk Assessment 2017; EFSAshort forEuropean Food Safety Authority 2019). An updated literature review yielded few new findings, which are briefly presented below together with the most important statements from previous opinions.
Petterson et al. and Stobiecki et al. used extracts from the seeds of L. angustifolius in their studies on acute toxicity and administered these to male Wistar rats and male and female mice. Oral LD50 values between 750 and >4,000 mgshort formilligram/kgshort forkilogram body weight were determined. One to 16 minutes after administration, the animals reacted with nervous symptoms such as tremors, followed by convulsions, cyanosis, collapse and death. Rats that survived the treatment showed no persistent clinical signs of toxicity (Petterson et al. 1987; Stobiecki et al. 1993).
For the isolated quinolizidine alkaloids lupanine and sparteine, LD50 values of 410 mgshort formilligram/kgshort forkilogram and 220 mgshort formilligram/kgshort forkilogram, respectively, were determined after oral administration to male Swiss mice. The symptoms, including tremors and tonic-clonic convulsions, were similar to those mentioned above (Yovo et al. 1984). An oral LD50 value of 1,664 mgshort formilligram lupanine/kgshort forkilogram is reported for male Wistar rats (Petterson et al. 1987).
Boschin et al. report that in 2016, more than 2,000 pigs were poisoned in northern Italy after accidentally being fed feed contaminated with alkaloid-rich lupin seeds for 2 to 13 days. The quinolizidine alkaloid content in the feed was between 51 and 1,245 mgshort formilligram/kgshort forkilogram. Symptoms ranged from severe refusal to eat to dilated pupils, increased salivation, recumbency and vomiting. Investigations into the 23 deaths revealed that gastric torsion or gastrointestinal bloating were the cause. An analysis of the feed to determine the profile of the quinolizidine alkaloids showed that most contained around 80 % lupanine, followed by 5-17 % multiflorine, 3-11 % albine and 3-11 % 13α-OH-lupanine (Boschin et al. 2022).
In a study conducted by Butler et al. (Butler et al. 1996), rats were fed for 3 months with feed containing approximately 2.9–6.6 mgshort formilligram lupin alkaloids/kgshort forkilogram body weight/day because of its flour content derived from L. angustifolius seeds. In addition, alkaloids were added to the feed of three groups, corresponding to an intake of 50 (control group), 250, 1,050 and 5,050 mgshort formilligram/kgshort forkilogram, respectively. The relative liver weights of female animals in the highest dose group showed a dose-dependent increase compared to those in the control group. Furthermore, altered foci of liver parenchyma cells (EFSAshort forEuropean Food Safety Authority 2012) and minor haematological changes were observed after 45 days of treatment, which, according to the authors, are not of biological significance.
In a 90-day study by Robbins et al. (Robbins et al. 1996), rats consumed 10, 30, 100, 500 mgshort formilligram lupin alkaloids/kgshort forkilogram BW/day in the form of an extract from L. angustifolius seeds in their feed. No haematological changes were observed. However, the authors describe a reduction in body weight in the two upper dose groups and derive a NOAEL of 30 mgshort formilligram/kgshort forkilogram BW/day on this basis. Significantly increased relative liver weights were observed in both sexes in the highest dose group and in males in the lowest dose group. Since the reduction in body weight could be solely due to reduced feed intake as a result of the bitter taste of the lupin alkaloids, the authors discuss whether a NOAEL of 100 mgshort formilligram/kgshort forkilogram BW/day would be more appropriate.
In a twelve-week feeding study in Sprague-Dawley rats, in which debittered seeds of L. mutabilis served as the sole source of protein, no adverse effects were observed (Schoeneberger et al. 1987).
In a 16-week feeding study with rats, Ballester et al. found no changes in feed intake, body weight development, organ weights or macroscopic and microscopic organ examinations compared to the control group when the feed consisted of more than half L. albus or L. luteus. According to the EFSAshort forEuropean Food Safety Authority, the estimated alkaloid intake was 26.6 and 42.3 mgshort formilligram alkaloids/kgshort forkilogram BW/day, respectively. The NOAEL derived from this is therefore the highest dose tested, corresponding to 26.6 mgshort formilligram L. albus alkaloids/kgshort forkilogram BW/day and 42.3 mgshort formilligram L. luteus alkaloids/kgshort forkilogram BW/day (Ballester et al. 1980; EFSAshort forEuropean Food Safety Authority 2012).
In a nine-month feeding study, the same research group found significantly reduced liver weights in rats fed a diet consisting of 51.8 % flour from L. albus (cultivar: Multilopa) (lupin content of the lupin meal: 0.025 %; estimated lupin intake: 11.7 mgshort formilligram lupanine/kgshort forkilogram BW/day). No other adverse effects were observed (Ballester et al. 1982; EFSAshort forEuropean Food Safety Authority 2012).
In addition, long-term feeding studies were conducted on animals, particularly rodents, with the main objective of investigating the nutritional benefits of L. angustifolius and L. albus seeds (Jecsai et al. 1986; Grant et al. 1993; Grant et al. 1995; Rahman 2000). In many cases, the alkaloid content of the seeds administered was not specified in these studies. These studies are therefore not suitable for assessing possible chronic toxicity or carcinogenicity in terms of their study design.
With regard to developmental and reproductive toxicity, Ballester et al. found that the only difference between the treated male and female animals and the control animals in the F1 and F2 generations of the nine-month feeding study described above was a significant reduction in relative liver weights (Ballester et al. 1982; Ballester et al. 1984).
3.2.2.1.4 Genotoxicity data
Lupanine and an alkaloid preparation from L. angustifolius proved negative in genotoxicity studies (bacterial mutagenicity test (Ames test) with and without metabolic activation) (Petterson 1998; Santiago Quiles et al. 2010). Due to the limited data available, the genotoxic potential of quinolizidine alkaloids cannot be assessed at this time.
In a more recent study by Schreiber et al., sparteine, lupinine, lupanine, 13α-OH-lupanine and angustifoline also showed no genotoxic activity in the Ames test and micronucleus test. However, the study has some limitations. For example, the highest concentration tested was 100 µM, at which no cytotoxicity was observed. Furthermore, the micronucleus test on HepG2 cells was only performed without a metabolic activation system (Schreiber et al. 2025).
3.2.2.1.5 Medicinal use of sparteine
(-)-Sparteine has been used in the past as an antiarrhythmic and oxytocic agent in medicines. In contrast to other quinolizidine alkaloids from lupin seeds, human data on the dose-response relationship of sparteine (sulfate) are available due to its pharmaceutical use, which are already described in detail in the EFSAshort forEuropean Food Safety Authority and BfRshort forGerman Federal Institute for Risk Assessment opinions (BfRshort forGerman Federal Institute for Risk Assessment 2017; EFSAshort forEuropean Food Safety Authority 2019). Therapeutic doses for sparteine sulfate are given here as 800 to 1,000 mgshort formilligram per day for acute treatment and 400 to 500 mgshort formilligram per day for chronic treatment of cardiac arrhythmias, with the daily dose divided into four to five single doses. For the treatment of tachycardia, single doses of 100 or 200 mgshort formilligram sparteine sulfate per tablet or ampoule were used. Antifibrillatory properties have also been reported at a dose of 20 mgshort formilligram.
The lowest dose identified by the EFSAshort forEuropean Food Safety Authority as the effective dose for antiarrhythmic effects in humans based on literature data is therefore 20 mgshort formilligram sparteine sulfate, which corresponds to 0.29 mgshort formilligram sparteine sulfate or 0.16 mgshort formilligram sparteine per kgshort forkilogram body weight for a 70 kgshort forkilogram adult (EFSAshort forEuropean Food Safety Authority 2019).
Sparteine is described as having a wide therapeutic range as an antiarrhythmic agent, with the therapeutic dose of sparteine sulfate being specified as a maximum of 4 mgshort formilligram/kgshort forkilogram body weight per single dose and toxic doses that can lead to respiratory paralysis or cardiac arrest being specified as 40 and 90 mgshort formilligram/kgshort forkilogram body weight, respectively. For infants, doses as low as 50 mgshort formilligram are considered toxic. For example, two infants died after taking 413 mgshort formilligram of sparteine Perivar tablets (Späth 1982; Blaschek et al. 2023).
Sparteine affects the nervous system and is described as a central stimulant in low doses, whereas higher doses can lead to peripheral respiratory paralysis, bradycardia and even cardiac arrest.
As an anticholinergic substance, it has a variety of undesirable effects and can lead to a characteristic complex of symptoms (anticholinergic syndrome), although in most cases only some of the peripheral and central symptoms are observed rather than the full picture of anticholinergic syndrome. Symptoms of poisoning after sparteine intake include dry mouth and skin, skin redness, dilated pupils (mydriasis) and accommodation disorders, bladder and bowel paralysis, hyperthermia, cardiac arrhythmia, hypertension, swallowing disorders, drowsiness, decreased strength in the legs, headaches, dizziness and concentration disorders, as well as changes in liver enzymes, for example (Thies 1986).
Sparteine was also used in obstetrics because of its oxytocin-like effect and was used to stimulate and intensify contractions. Single doses of up to 150 mgshort formilligram were reported, with total doses of up to 600 mgshort formilligram being used. Sparteine sulfate was injected intramuscularly. According to reports, the use of sparteine sulfate as a labour-inducing agent led to foetal deaths and uterine ruptures. The complications that occurred were comparable to those of intravenously administered oxytocin (Newton et al. 1966). Individual differences, both in terms of therapeutic effect and side effects, can be explained in part by the genetic polymorphism of the CYP2D6 isoenzyme (see 3.2.2.1.1).
In addition, sparteine has also been discussed as a potential active ingredient for the treatment of venous disorders (Kreuzer & Lüth 1959).
In folk medicine, broom (Cytisus scoparius) is used to treat cardiovascular complaints or hypotension due to its high content of quinolizidine alkaloids (up to 1.5 % in the seeds, up to 0.8 % in the leaves) with the main alkaloid sparteine. Here too, symptoms of poisoning such as nausea, abdominal pain, diarrhoea, cramps and vomiting, circulatory collapse and cardiac arrhythmia have been described (Wink et al. 1983; Chrubasik-Hausmann 2022; Informationszentrale gegen Vergiftungen NRW 2024).
3.2.2.1.6 Cases of poisoning and case reports following consumption of foods containing lupin seeds
It should be noted that the symptoms after consuming lupin seeds with high alkaloid content can vary greatly and affect the digestive tract, the cardiovascular system and the nervous system. Typical symptoms of moderate poisoning include dilated pupils, dizziness, nausea, dry mouth, stomach pain, vomiting, diarrhoea and/or heart problems. These physical symptoms, some of which are very unspecific, cannot always be attributed to a clear cause, as they can also occur in other diseases. It can therefore be assumed that some cases of poisoning or physical complaints after consuming foods containing lupin seeds go undetected or unreported. Severe symptoms following poisoning with quinolizidine alkaloids are often characterised by curare-like paralysis and convulsions, and can even lead to death as a result of respiratory paralysis, suffocation or cardiac arrest (Schmidlin-Mészáros 1973).
Details of cases of poisoning associated with the consumption of lupin seeds and published before 2017 are described in earlier BfRshort forGerman Federal Institute for Risk Assessment and EFSAshort forEuropean Food Safety Authority opinions (BfRshort forGerman Federal Institute for Risk Assessment 2011, 2017; EFSAshort forEuropean Food Safety Authority 2019).
Reports from German poison information centres (GIZ)
The BfRshort forGerman Federal Institute for Risk Assessment conducted a survey of German poison information centres on cases of exposure to lupins (and lupin products) for the period 2016–2021.
Four of the seven German GIZs responded to the GIZ survey (Table A1 in the appendix). These GIZs cover approximately 50 % of the enquiries received by German GIZs. A total of 160 cases were documented during the six-year period covered by the survey. In the previous comparison period (2010–September 2015), a total of 130 exposures were reported by (at that time) eight out of eight GIZs (100 % of enquiries to German GIZs).
Table A1 in the appendix shows that 76 % (107/141) of oral exposures in the last survey period from 2016 to 2021 were caused by the ingestion of plants and 9 % (12/141) by the ingestion of food. In contrast, in the period from 2010 to September 2015, 92 % of oral exposures were due to the ingestion of plants and only 6 % to the ingestion of food.
In terms of the severity of poisoning, 78 % of exposures were asymptomatic. In 18 % of cases, mild symptoms were recorded and in 3 % moderate symptoms. Between 2010 and September 2015, 76 % of cases were asymptomatic and 24 % had mild symptoms. No cases with moderate or severe symptoms were reported in the earlier period.
Two of the seven GIZs provided detailed information on individual cases (Table A2 in the appendix). A total of 94 exposures were reported, 85 of which involved plants and nine of which involved food. The most common form of exposure was oral ingestion of lupin seeds and pods.
Seventeen cases with mild symptoms and four cases with moderate symptoms were reported (severity (PSS) 2 (moderate symptoms)External Link:[1]: 2× seed decoction, 1× yoghurt, 1× contact with plant sap). The most commonly reported symptoms were dizziness, nausea with vomiting and abnormal sensations.
The age group most frequently represented here was infants (40 exposures), followed by adults (27 exposures) and schoolchildren (17 exposures).
In summary, extrapolating the reported cases of four GIZs to the possible number of enquiries for all GIZs (estimated at 320 enquiries) shows a significant increase compared to the previous survey period (2010 – September 2015). However, the proportion of exposures involving food has increased only slightly, with exposure to plants continuing to dominate. The proportion of asymptomatic cases has remained almost unchanged. In the current period, however, cases with moderate symptoms (PSS 2) were also reported. One case was clearly linked to the consumption of a food product (lupin yoghurt). In addition, two patients showed mild symptoms (PSS 1, including dizziness, nausea, coordination disorders) after consuming lupin patties (see Table A2 in the appendix).
It should be noted that only some of the GIZs responded to the survey and that the data was retrieved retrospectively. The GIZ was therefore only able to identify cases that contained the root word “lupin” in the name assigned to the toxic substances by the GIZ, but not, for example, products containing lupin seeds that were stored without the root word lupin. Data is collected according to medical/toxicological criteria, which is why certain details, such as the exact species name of plants, are not necessarily documented. The majority of cases cannot be traced by the GIZ. Therefore, the severity usually corresponds to that recorded at the time of the (last) call.
Reports from the BfR case database (national)
The following cases of adverse effects associated with exposure to lupins or lupin preparations have been reported to the BfRshort forGerman Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment case database) for the period 2016–2021:
- One adult female: consumption of a casserole topped with vegan cheese made from lupin flour. This resulted in severe gastrointestinal symptoms (vomiting, diarrhoea) and headaches (BfRshort forGerman Federal Institute for Risk Assessment: classified as moderate severity). Other people who had also eaten the dish showed no symptoms.
- Two individuals (mother and daughter (13 years old)): consumption of sweet lupin meal in a vegetable dish. An unpleasant taste was noticed. Daughter: brief vomiting (BfRshort forGerman Federal Institute for Risk Assessment: classified as mild severity), mother: severe vomiting and headaches over several days (BfRshort forGerman Federal Institute for Risk Assessment: classified as moderate severity). Lupin meal was examined by the BfRshort forGerman Federal Institute for Risk Assessment: the sample had a quinolizidine alkaloid content slightly above the maximum value of 200 mgshort formilligram/kgshort forkilogram quinolizidine alkaloids for lupin products set by various countries. The pronounced gastrointestinal symptoms cannot be explained by the measured quinolizidine alkaloid content.
- When the above cases were presented at the meeting of the BfRshort forGerman Federal Institute for Risk Assessment Commission for the Assessment of Poisonings in November 2021, the BfRshort forGerman Federal Institute for Risk Assessment was informed of another case with moderate symptoms in which water used to remove the bitterness from white lupins was accidentally drunk (this is very likely to be a case also reported by “GIZ 1” in Table A2 in the appendix).
Reports from free literature search (international)
The BfRshort forGerman Federal Institute for Risk Assessment has researched reports of poisoning cases or cases with adverse effects related to the consumption of foods containing lupin seeds and lupin seeds for the period from 2017 to July 2024 and listed details of the identified reports below.
- 63-year-old man and his wife: consumption of approximately 300 mlshort formillilitre and 100 mlshort formillilitre of water, respectively, in which lupins (from Ecuador) had been soaked for several hours. The husband vomited several times 15 minutes after consumption. Repeated visits to the casualty department due to the sudden onset and subsequent easing of various symptoms of anticholinergic syndrome (difficulty urinating, confusion, hallucinations, flatulence, dry skin, dilated pupils) at different times up to 15 hours after exposure. The wife showed similar symptoms (change in vision, anxiety, digestive problems, dizziness, nausea, weakness). Both individuals asymptomatic after 24 hours. Laboratory measurement of lupanine and sparteine levels in the source of exposure and patient sera (2 hours after exposure): soaking water: 3.1 mgshort formilligram/mlshort formillilitre lupanine, 0.89 mgshort formilligram/mlshort formillilitre sparteine; husband’s serum: 170 ng/mlshort formillilitre lupanine, <1 ng/mlshort formillilitre (LODshort forLimit of detection) sparteine; wife’s serum: 71 ng/mlshort formillilitre lupanine, <1.3 ng/mlshort formillilitre sparteine. According to the authors, there is a correlation between the severity of symptoms and the measured serum levels (Lishort forlithium et al. 2017).
- One-year-old boy (possibly from Peru): ingestion of an unprocessed and raw lupin seed (L. mutabilis Sweet, “chocho”) 3 hours before admission to hospital. Initial symptoms: acute respiratory distress during sleep, bluish discoloration around the mouth, severe cough. Endoscopy: lupin seeds in the stomach (entrance). Six hours after admission: altered consciousness, shallow breathing, dilated pupils and dry mucous membranes, distended abdomen. After 24 hours, improved mental status; after 48 hours, watery stools accompanied by elimination of the seed, no neurological signs; after 72 hours, discharge.(Flores-Pamo et al. 2018)
- 48-year-old man (Argentina): ingestion of homoeopathic medicine for osteoarthritis with presumably high amounts of L. mutabilis. Symptoms (after 6 hours) upon admission to the casualty department: excessive pupil dilation (bilateral hyporeactive mydriasis), blurred vision, dizziness, palpitations. After 12 hours, normal pupil dilation (asymptomatic) (Alessandro et al. 2017).
- 56-year-old woman (from Italy, case in France): consumption of two handfuls of lupin seeds (incompletely processed). Symptoms after one hour: nausea, vomiting, dizziness, blurred vision, dry mouth. After 7 hours, contact with the casualty department, admission, hydration and discharge after a few hours (Schmitt et al. 2019).
- 38-year-old man (France): consumption of cooking water from lupin seeds. Symptoms after 3 hours: vomiting, dizziness, blurred vision, urinary retention. Admitted to the casualty department after a few hours: anuria treated with intravenous fluids and catheter. Discharged (asymptomatic) the next day. No known medical history (Schmitt et al. 2019).
- 39-year-old man (Portugal): consumption of a large quantity of lupin seeds harvested and prepared by himself. Symptoms appeared 3 hours later and he was admitted to the casualty department (9 hours after consumption): blurred vision, dry eyes and mouth, anxiety, fixed bilateral pupil dilation without accommodation reflex, restlessness. Full recovery 16 hours after the onset of symptoms. No known medical history (Silva et al. 2020).
- 73-year-old man: consumption of slightly soaked lupin seeds. Shortly afterwards, dry throat, dry mouth, dizziness and vomiting. Admitted to casualty department: confusion, irregular heart rhythm, anxiety, dry mouth, dilated pupils. Treated with benzodiazepine. Discharged (asymptomatic) the next day. His wife also consumed these lupin seeds: no symptoms described (Agnew et al. 2020).
- 40-year-old woman (possibly Jordan): consumption of bitter lupin seeds boiled for 15 minutes (20 seeds, ≈ 60 g). Symptoms developed after 30 minutes. Admitted to the casualty department with the following symptoms: blurred vision, nausea, abdominal pain, dizziness, disorientation, sinus tachycardia, facial flushing, dry mouth, reduced bowel sounds, fixed, dilated, unresponsive pupils on both sides. Asymptomatic within 24 hours. No known pre-existing conditions or allergies (Al-Abdouh et al. 2020).
- 50-year-old woman (Lebanon): ingestion of partially debittered lupin seeds and rapid onset of symptoms. Admitted to hospital after a few hours. Symptoms: excessive bilateral pupil dilation, dry mouth, anxiety. Asymptomatic 12 hours after admission. Husband: similar symptoms (without pupil dilation), stomach pain, general malaise (Lahoud et al. 2021).
- 12-year-old boy (possibly Turkey): tonic-clonic (epileptic) seizures and vomiting 2 hours after consuming raw, undebittered lupin seeds (two handfuls, ≈ 300 g, from the family farm). Admitted to the casualty department and transferred to the intensive care unit 6 hours after consuming the seeds. Treated with midazolam and fentanyl. Symptoms: bilateral pupil dilation with weak light reflexes, increased muscle tone, hot flushes, goose bumps, dry mouth, bilateral Babinski reflex, tachycardia, high blood pressure, tachypnoea, Glasgow Coma Scale score of 12 (assessment: mild disturbance of consciousness). Condition normalised after 5 days. No previous allergic reactions or symptoms of poisoning from lupin seeds known in the boy (Ozkaya et al. 2021).
- 52-year-old woman (Ecuador): consumption of water from soaked lupin seeds. Malaise and dizziness a few minutes after consumption. Admitted to the casualty department (30 minutes after consumption) with the following symptoms: visual disturbances, dry mouth, sensitivity to light, impaired vision, difficulty swallowing, dry eyes, nausea, low blood pressure, non-reactive pupil dilation. Serum therapy and treatment with antiemetic and activated charcoal. Discharged (asymptomatic) after 24 hours. No known allergies or medical history (Esparza et al. 2021).
- 49-year-old woman: consumption of one cup of insufficiently debittered lupin seeds. Shortly after ingestion: dizziness and dry mouth as well as speech problems. Admission to the casualty department: non-reactive pupil dilation and confusion observed. Symptoms improved within 6 hours and patient discharged. No known medical history (Alsakha & Eggleston 2023).
- 56-year-old woman (France): consumption of handmade flour (10 g/day) made from lupin seeds over a period of more than one year. Occurrence of muscle fasciculation and cramp (cramp-fasciculation syndrome (CFS)) and pupil dilation over a period of 18 months with varying daily intensity. The reported time between consumption and the onset of symptoms was one month. Complete remission of CFS symptoms after four months of avoiding foods containing lupin seeds (Lagrange et al. 2024).
- 41-year-old male twins (France): 5-days’ consumption of flour (200 g/day) made from raw lupin seeds as the sole source of nutrition (high in protein) during a hike. Occurrence of dilated pupils and nausea within one day of consumption. Decrease in (CFS) symptoms 15 days after discontinuing the diet containing lupin seeds (Lagrange et al. 2024).
3.2.2.2 Allergenic potential of lupin seeds
3.2.2.2.1 Lupin allergy and prevalence
The first well-documented report of lupin allergy was published in 1994, describing the case of a 5-year-old girl who developed symptoms of hives and angioedema after eating spaghetti to which lupin flour had been added during processing (Hefle et al. 1994). Shortly afterwards, a case of anaphylactic reaction specifically to lupin flour from Spain was reported (Matheu et al. 1999). In both cases, the patients were confirmed peanut allergy sufferers. At that time, it was still unclear whether all allergies elicited by lupin seeds belonged to the category of cross-allergies or whether primary sensitisation and elicitation reactions specific to lupin allergens could also occur. Recent studies have now established that the latter can also be the case (Peeters et al. 2007). Lupin allergens can therefore induce primary sensitisation and, in the case of existing allergies to other legumes, elicit allergic symptoms as cross-allergens. In clinical studies in which only lupin-specific IgE is tested for (e.g. multiplex blood test) and for which no additional data from provocation tests are available, the question of primary sensitisation cannot be clarified beyond doubt. According to a report by the University of Miami, a multiplex blood test (ImmunoCAP) found concentrations of specific IgE in the range of 0.3 to 3.3 kilounits (kU)/litre (L) in patients in response to lupins (Muffly et al. 2022).
To investigate possible differences in reactions to different lupin species, a recent study produced protein extracts from L. albus, L. angustifolius and L. luteus and compared the reactions (Aguilera-Insunza et al. 2023). Specific IgE protein reactivity was found in all three species, with no significant differences apparent.
Parallel to the increase in the consumption of legumes over the last twenty years, cases of allergies caused by legumes have also become more frequent. In children in France, legumes are responsible for 15 % of all anaphylactic reactions (Muller et al. 2022). Also in France, the country where lupin flour was approved as an additive to wheat flour in 1997, lupin flour ranks fourth among allergy-eliciting foods after peanuts, nuts and shellfish, according to reported food allergies associated with anaphylactic symptoms (Moneret-Vautrin et al. 2004).
In another report by the Allergy Vigilance Network, 62 cases (2.3 %) of the 2,708 documented cases of food-induced anaphylaxis between 2002 and 2020 were diagnosed as lupin allergy (Pouessel et al. 2024). It was found that, unlike in adults, there was a significant increase in cases among children during the reporting period. Lupin-induced anaphylaxis was most commonly associated with sensitisation to soy, pistachio and hazelnut. Broken down by families of molecular components, lupin allergy occurred with reactions to storage proteins, Bet v 1-homologous proteins (PR-10), lipid transfer proteins and profilins in that order. In the study from France and Belgium, the food products most commonly associated with lupin-induced anaphylaxis cases were cakes, biscuits, waffles and bread. However, lupin flour as a food ingredient is often not immediately obvious to consumers and even to treating allergists as the cause of allergic reactions. For example, a scientific case report published by Münster University Hospital described the case of a patient with oropharyngeal symptoms and dyspnoea after consuming a rum ball. Lupin flour as the cause of the symptoms could only be identified after provocation with the food, a manufacturer’s enquiry and immunoblot testing for primary sensitisation to lupin (Grundmann et al. 2008).
When assessing the risk of allergies caused by food, information on the extent to which the processing of the product alters its allergenic properties is highly relevant to consumers. In the case of lupin, there are only a few scientific studies available on this subject. Lupin proteins with allergenic properties are largely heat-stable. However, according to a recent study, a roasting temperature of over 195 °Cshort fordegrees Celsius drastically reduced the detectability of lupin protein from seeds in ELISA (enzyme-linked immunosorbent assay) and other analytical methods (Beyer et al. 2024). Consequently, it can be assumed that the roasting process simultaneously reduces allergenic potency. The allergen binding mechanisms via epitope recognition by antibodies are comparable in the analytical method ELISA and in the IgE-mediated elicitation reaction in humans by memory cells. It is therefore scientifically plausible that data from various analytical methods by Beyer, which showed an allergen-attenuating effect of the roasting temperature on lupin seeds, could be transferred to human reactions in the body. A significant reduction in the allergenicity of lupin seeds was demonstrated by high-pressure sterilisation (autoclaving at 138 °Cshort fordegrees Celsius) via IgE immunoblotting and skin prick testing. However, according to this study, autoclaving at 121 °Cshort fordegrees Celsius, microwave heating or extrusion cooking at 135 °Cshort fordegrees Celsius is not sufficient (Álvarez-Álvarez et al. 2005).
3.2.2.2.2 Symptoms and severity
The symptoms of an allergic reaction to lupin vary from person to person and can range from oral allergy syndrome to anaphylactic reaction, depending on the clinical classification, with the highest severity being 3 (“severe”) or 4 (cardiovascular and CNS symptoms) (Jappe & Vieths 2010; Trautmann & Kleine-Tebbe 2022). These allergy symptoms are mainly located in the oral, dermal and gastrointestinal tracts and include hives, angioedema, inflammation of the nasal mucosa and conjunctiva (rhinoconjunctivitis), lip or other oedema, respiratory distress (laryngospasm, dyspnoea, asthmatic attacks), nausea, mild to severe abdominal pain and, in some cases, anaphylactic shock. One such case in Germany was reported by the University Dermatology Clinic in Heidelberg. A 52-year-old patient suffered facial and tongue oedema, dizziness and breathing problems after eating a nut croissant and had to be treated in hospital (Brennecke et al. 2007). In addition to IgE against lupin seeds and positive results in the prick test against lupin flour, specific protein detection via Western blot was able to diagnose monovalent sensitisation to lupin flour in this case, i.e. no cross-reaction.
In the clinical literature, the severity of a lupin allergy is stated to be comparable to that of a peanut allergy (Peeters et al. 2007). A group of experts from the Codex Alimentarius (FAO & WHO 2022) classified lupins, together with peanuts, as “Group B: higher proportion of anaphylaxis in 1-2 regions”. Clinically diagnosable anaphylactic symptoms have already been caused by low to moderate eliciting doses (ED) in people with lupin allergy in various studies.
A BfRshort forGerman Federal Institute for Risk Assessment publication (BfRshort forGerman Federal Institute for Risk Assessment 2011) evaluated thirteen case reports of patients who had consumed lupin products. In these cases, the symptoms ranged from mild to life-threatening (including rhinitis, urticaria, shock). The time to symptom onset was very short, e.g. only a few minutes after consuming three lupin seeds (Matheu et al. 1999). In these summarised reports, elicitation reactions were described not only via the oral route, but also via inhalation in five cases and via the skin of the lips, i.e. via the dermal route, in one case. One case of anaphylactic syndrome in an Australian woman after consuming a roll with lupin bran (Smith et al. 2004) and cases of anaphylactic shock in a child and a 25-year-old woman after consuming pizza and dough containing lupin flour, respectively, have been documented (Leduc et al. 2002; Radcliffe et al. 2005). In the evaluation of all case reports (BfRshort forGerman Federal Institute for Risk Assessment 2011), the symptoms urticaria, angioedema and rhinoconjunctivitis were recorded in this order of frequency of occurrence in the affected persons.
In a recent study involving 43 individuals with peanut allergy, 23 patients were found to have a lupin allergy in a provocation test, with anaphylaxis reported as the most common manifestation after consumption of lupin flour (Aguilera-Insunza et al. 2023).
3.2.2.2.3 Cross-reactivity
Allergic cross-reactions can occur when an immunoglobulin of class E recognises molecular target structures (epitopes) of different allergens on different foods, in this case proteins from different legumes, that are very similar or identical in terms of amino acid sequence and spatial structure. The frequency of cross-reactions for lupin, clover and shrimp is rated as “rare”.
On the other hand, lupin is now the legume most frequently studied in allergy studies after peanuts. Nevertheless, the prevalence of cross-reactivity to peanuts found in various studies varies from 3 % to 30 % (Matheu et al. 1999; Reis et al. 2007; Shaw et al. 2008; Cousin et al. 2017).
In the early case report of lupin allergy after consumption of pasta (Hefle et al. 1994), seven other individuals were also tested for skin reactions using an extract of sweet lupin seed pasta in a prick test and additionally for specific IgE using lupin seeds in a radioallergosorbent test (RAST). The results showed that five of the seven individuals with peanut allergy had a positive reaction to the extract in the skin test. In addition, four of the seven peanut-sensitive patient sera showed IgE binding to the sweet lupin seed pasta extract in the RAST. The very high frequency of cross-reactivity to peanuts observed here may also be coincidental and due to the small number of cases. A well-documented case study from England reports that lupin seeds processed in food can elicit severe allergic reactions in people with peanut allergy (Radcliffe et al. 2005). Just 15 minutes after eating a meal accompanied by onion rings prepared with a batter made from lupin flour, a restaurant patron experienced severe anaphylactic reactions with respiratory and circulatory problems. The prick test was strongly positive for peanuts and lupins, and specific IgE was detected. At that time, the exclusion diagnosis of idiopathic anaphylaxis often could not be refuted by specific tests for lupin flour allergy, and therefore a lupin allergy could not be diagnosed independently of cross-reactivity to peanuts.
In a study on the cross-reactivity of lupin and peanut, five out of eight peanut-allergic individuals responded to lupin flour with allergic symptoms in double-blind, placebo-controlled, oral provocations (Moneret-Vautrin et al. 1999). In further studies, sera from individuals who were allergic to lupin protein showed no binding to the peanut allergens Ara h1-h3.
In an experimentally well-validated study on lupin allergy, prick tests, CAP tests and double-blind, placebo-controlled provocation tests were performed together to distinguish cross-allergies to peanuts from primary sensitisation (Peeters et al. 2007). This showed that three patients had co-sensitisation to peanuts and lupin flour. However, two patients reacted to lupin alone, meaning that there was no cross-allergy in this case.
In a larger prospective study involving patients from France and Belgium in two age groups, individuals with peanut allergy were tested for cross-reactivity using a skin prick test with lupin extract (Gayraud et al. 2009). In patients with peanut allergy, the frequency of cross-reactivity with lupin was 17 % in children under 16 years of age and 15 % in adults.
In an earlier study, the authors (Leduc et al. 2002) found a significantly higher cross-reactivity of 68 % of individuals with peanut allergy who were hypersensitive to lupin protein, but not with the skin prick test used in the study by Gayraud et al.
Another study investigated the frequency of co-sensitisation to various legumes (Smits et al. 2023). For people allergic to lupins, co-allergy to peanuts and soybeans was frequently observed (>50 %), with 7S and 11S globulins being primarily responsible for co-sensitisation. After oral provocation, another study confirmed lupin allergy in 44 % of people with peanut allergy (Aguilera-Insunza et al. 2023).
A recent retrospective study investigated cross-reactions in 195 children with peanut allergy and documented consumption of or sensitisation to at least one other legume (Muller et al. 2022). The study recorded consumption history, skin prick test results, specific IgE levels, previous allergic reactions and results of provocation tests for other legumes. In the investigations of consumption, sensitisation and legume allergy, lupin was identified as the most frequently evaluated legume, at 95 %. A further finding was that 64 % of the children examined with peanut allergy were sensitised to at least one other legume, 34 % of them to lupin. Lentils, lupins and peas were identified as the main allergens, with 5 % of children having multiple legume allergies. Finally, an oral provocation test determined a prevalence of 19 % for cross-allergy to lupins in sensitised children. In addition to the high prevalence, the proportion of severe allergic reactions was also determined. The Astier’s Score used shows that 50 % of children with severe anaphylactic reactions reacted to lupin. The significance of this study is limited by the fact that consumption and sensitisation to the various legumes were recorded separately.
3.2.3 Exposure
3.2.3.1 Data on the consumption of foods containing lupin seeds
In its 2019 opinion, the EFSAshort forEuropean Food Safety Authority concludes that the data on the consumption of foods containing lupin seeds is very limited (EFSAshort forEuropean Food Safety Authority 2019). Sweet lupin cultivation in Germany has increased steadily over the last 10 years (BMELshort forGerman Federal Ministry of Food and Agriculture 2023). Market research on legumes (including soybeans) conducted by the Federal Information Centre for Agriculture (BZL) indicates that domestic lupins accounted for an estimated 7.5 % of legumes used for food in 2022 (BLE 2023).
A search of the Mintel databaseExternal Link:[2] shows that over the last 10 years, around 20 to 44 food products containing lupin seeds or lupin seed flour have been launched on the German market each year, more than half of which fell into the baked goods category. This underlines that cereal flours can be replaced or supplemented by lupin flours in the production of baked goods and pasta, for example (DGE 2016).
It can be assumed that consumption will continue to increase on the German market due to the increased demand and supply of products containing lupin seeds, especially as substitutes for meat and dairy products, but also as alternatives to soy products and cereal flours.
3.2.3.2 Data on the occurrence of quinolizidine alkaloids in food
A data query with the Federal Office of Consumer Protection and Food Safety (BVLshort forGerman Federal Office of Consumer Protection and Food Safety) and the Federal State investigation offices for the years 2019 to spring 2022 showed that, up to that point, data on a limited number of different quinolizidine alkaloids (lupinine, lupanine, 13α-OH-lupanine, sparteine and angustifoline) in food had been collected. The following ten food groups were examined: bread and pastries, ice cream and ice cream products, honey/beekeeping products and spreads, legumes/oilseeds and nuts, teas and tea-like products, coffee substitutes and additives, vegan/vegetarian substitutes, cheese, milk and dairy products. However, the QA levels determined were subject to significant variation, which can vary in severity depending on the type of food and the analyte under consideration, among other factors. Overall, it was found that the sample numbers were often too small to make valid statements about the distribution of the levels, which is why the BfRshort forGerman Federal Institute for Risk Assessment supported further investigations as part of the project monitoring.
3.2.3.2.1 Content data from the 2022 and 2023 project monitoring by the Federal and State Governments
In the annual monitoring measures carried out by the Federal and State Governments in accordance with sections 50-52 of the Food and Feed Code, various lupin products from Germany and the EU (vegan/vegetarian substitutes for meat products based on lupins, lupin flour, lupin meal and lupin coffee) were tested for the first time in 2022 for a total of ten quinolizidine alkaloids (lupinine, lupanine, sparteine, angustifoline, 13α-OH-lupanine, isolupanine, anagyrine, multiflorine, thermopsine and cytisine).
In the 48 samples measured, the levels of lupinine, lupanine, sparteine, angustifoline and 13α-OH-lupanine were determined in almost all samples, while only a few results were available for the other parameters. The main alkaloids found included lupanine and 13α-OH-lupanine (BVLshort forGerman Federal Office of Consumer Protection and Food Safety 2023).
As part of the 2023 monitoring programme, cow’s milk was tested for a total of eleven quinolizidine alkaloids (lupinine, lupanine, sparteine, angustifoline, 13α-OH-lupanine, isolupanine, anagyrine, multiflorine, thermopsine, cytisine and albine) for the first time. Quantifiable levels, which are far lower than in foods based on lupin seeds, were measured in 47 of a total of 73 samples. The main alkaloids in the samples tested were lupanine and 13α-OH-lupanine, followed by isolupanine, angustifoline and multiflorine. It was found that cow’s milk samples in accordance with EU Organic Regulation 2018/848 had higher overall levels than milk samples from conventional animal husbandry, although it should be noted that the number of samples was small and therefore the data pool is not meaningful (BVLshort forGerman Federal Office of Consumer Protection and Food Safety 2024).
Figure 1 shows the results of the total values of quantifiable quinolizidine alkaloids in the products examined as part of the 2022 and 2023 monitoring programmes. A comparison of the respective maximum value with the median value shows that quinolizidine alkaloid levels can vary greatly depending on the sample examined from the same food group.
Monitoring 2022 - Total alkaloid content
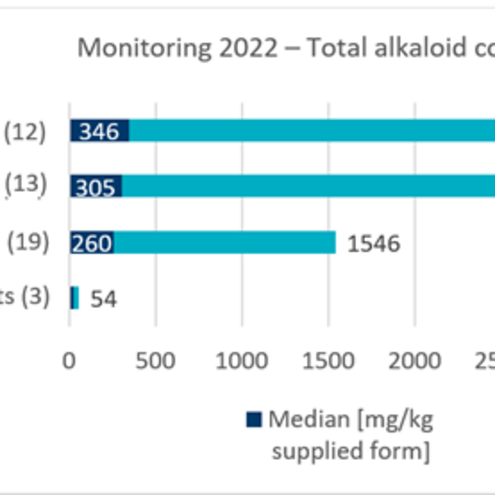
[Translate to Englisch:] Monitoring 2023 - Total alkaloid content

3.2.3.2.2 Selection of further data on quinolizidine alkaloid content from the literature
Keuth et al. examined 30 lupin seed-based food products from German retailers (North Rhine-Westphalia) between 2019 and 2021. Values of 20,000 and 21,000 mgshort formilligram/kgshort forkilogram were found in two samples of bitter lupin seeds. In samples of commercially available sweet lupin seeds, lupin flour and lupin meal, total alkaloid contents in the three-digit range (113–609 mgshort formilligram/kgshort forkilogram) were determined, whereas here too, lupanine and 13α-OH-lupanine were among the main alkaloids in terms of quantity. Angustifoline, sparteine and lupinine were also detected in double-digit mgshort formilligram/kgshort forkilogram quantities. In a sample of coffee substitute containing lupins, a total alkaloid content of around 1,200 mgshort formilligram/kgshort forkilogram was determined, with 13α-OH-lupanine as the main alkaloid (1,049 mgshort formilligram/kgshort forkilogram). Other product groups such as milk and yoghurt substitutes, savoury spreads and bread containing lupin seeds as an ingredient had only a low content of quinolizidine alkaloids (<50 mgshort formilligram/kgshort forkilogram) (Keuth et al. 2023).
In a study conducted by Hwang et al. in Korea in 2020, seeds of narrow-leaved lupin (L. angustifolius) purchased online (May–August 2018) and foods containing lupin seeds were tested for their content of five quinolizidine alkaloids (lupanine, 13α-OH-lupanine, angustifoline, sparteine and lupinine). In the nine samples of raw seeds analysed, total alkaloid contents of 440 to 57,157 mgshort formilligram/kgshort forkilogram were measured, with lupanine as the main alkaloid. Total alkaloid contents in the range of 13–1,375 mgshort formilligram/kgshort forkilogram were determined in bean patties, flours (“baking powder”) and pickled preserves (“prickles”). Very low alkaloid contents were quantified in noodles, biscuits and milk substitutes (“bean milk”) (<50 mgshort formilligram/kgshort forkilogram) (Hwang et al. 2020).
As part of a priority campaign in 2022, AGES investigated the quinolizidine alkaloid content of 26 commercially available products containing lupin seeds. Low total quinolizidine alkaloid contents of <5 mgshort formilligram/kgshort forkilogram were determined for processed products (e.g. ice cream or desserts), whereas high levels of 1,240.2 mgshort formilligram/kgshort forkilogram and 1,208.6 mgshort formilligram/kgshort forkilogram were detected in one sample of lupin flour and one sample of lupin coffee (powder), respectively, presumably due to the use of bitter lupin seeds (AGES 2022).
There was recently a report of a product containing lupin seeds offered for sale on the German market with a total content of quinolizidine alkaloids of 22,283 mgshort formilligram/kgshort forkilogram in the European Rapid Alert System for Food and Feed (RASFF) (05.02.2024, No. 2024.0777). A content of 15,940 mgshort formilligram/kgshort forkilogram for lupanine and of 76.9 mgshort formilligram/kgshort forkilogram for sparteine was determined (European Commission 2024).
Schryvers et al. investigated the effect of different processing methods on the content of quinolizidine alkaloids (sparteine, lupanine, lupinine, 13α-OH-lupanine and angustifoline) in lupin seeds from Lupinus albus (according to the manufacturer, a batch of sweet lupins with a relatively high quinolizidine alkaloid content) and foods produced from them. The lupin seeds examined in the study and used to produce the foods came from Germany (Bavaria) and were harvested in 2021. The total alkaloid content in the dry matter of the raw seed flour was 1,430 ± 80 mgshort formilligram/kgshort forkilogram, with lupanine accounting for the largest proportion of the quantified quinolizidine alkaloids. In addition, other alkaloids were identified in the raw material using another method (HRMS – high-resolution mass spectrometry) (cytisine, 13-angeloyloxylupanine or 13-tigloyloxylupanine, 17-oxolupanine, albine, multiflorine), although no quantification was performed here. A large difference in total alkaloid content was found between pods (170 ± 30 mgshort formilligram/kgshort forkilogram) and the podded seeds (1,600 ± 300 mgshort formilligram/kgshort forkilogram). The authors investigated the change in quinolizidine alkaloid content in lupin seeds when these were steam-cooked and then dried or treated under high pressure, autoclaved and then dried (referred to by the authors as “toasting”). This showed that the total alkaloid content in the flour and in the podded seeds was reduced by 11 to 23 %. Preserved lupin seeds, which had been soaked for 24 hours, boiled at 100°C for 30 minutes and then preserved in brine and sterilised, had a total alkaloid content of 270 ± 3 mgshort formilligram/kgshort forkilogram, a reduction of 63 %. The autoclaving process at 121°C had the greatest effect, whereas soaking in water alone only slightly reduced the concentration of total alkaloids but interestingly resulted in a higher content of 13α-OH-lupanine (+103 %), angustifoline (+21 %) and sparteine (+3 %) and a decrease in lupanine (-12 %), suggesting a conversion of lupanine to 13α-OH-lupanine in water. Boiling alone reduced the total content by only 28 %, although high variability is assumed here due to the possible transfer of alkaloids into the cooking water from the cells damaged by the boiling process. The alkaloid content in products such as lupin biscuits, crisps and pasta was also investigated. Pasta dough with a lupin content of 22 % had a quinolizidine alkaloid content of 320 ± 10 mgshort formilligram/kgshort forkilogram. In the cooked pasta, the content was reduced by 52 %, presumably due to the transfer of alkaloids into the cooking water. In biscuit dough with 14 % added lupin flour, the quinolizidine alkaloid content (270 ± 10 mgshort formilligram/kgshort forkilogram) was reduced by 15 % after baking the biscuits at 205°C for 9 minutes. A total content of quinolizidine alkaloids of 1,190 mgshort formilligram/kgshort forkilogram was measured in crisps with a lupin flour content of 60 %, the QA content falling by 19 % after deep-frying in vegetable oil (Schryvers et al. 2023).
Animal products are considered another source of exposure, as they may contain quinolizidine alkaloids transferred from feed. A study conducted by the BfRshort forGerman Federal Institute for Risk Assessment in 2022 published findings on the transfer of quinolizidine alkaloids from narrow-leaved lupins (L. angustifolius) to the milk of dairy cows. Four dairy cows were fed one kilogram of sweet lupin meal daily for seven days and then, after a ten-day break, another two kilograms of sweet lupin meal, each with a known content of lupin alkaloids in the ration. The transfer rate of quinolizidine alkaloids into milk was 0.13 % for sparteine, 1.74 % for 13α-OH-lupanine, 2.31 % for lupanine and 3.74 % for multiflorine (Engel et al. 2022).
Another study by Schryvers et al. investigated the possible transfer of quinolizidine alkaloids from feed containing lupin seeds (L. angustifolius) for calves into the animals’ meat. The animals were fed feed containing lupin seeds for four months. An average quinolizidine alkaloid content of 40 ± 30 mgshort formilligram/kgshort forkilogram was determined in the compound feed samples. The main components were lupanine (57 %), 13α-OH-lupanine (32 %) and angustifoline (9 %). The quinolizidine alkaloid content was 9–14 µgshort formicrogram/kgshort forkilogram in the muscle meat samples and 51–62 µgshort formicrogram/kgshort forkilogram in the liver samples. In order to also detect metabolites, the samples were subjected to enzymatic hydrolysis with β-glucuronidase and arylsulfatase. As a result, a quinolizidine alkaloid content of 73–84 µgshort formicrogram/kgshort forkilogram was determined in the muscle meat samples and 106–117 µgshort formicrogram/kgshort forkilogram in the liver samples (Schryvers et al. 2024b).
3.2.4 Conclusions
3.2.4.1 Evaluation of the data available on the toxicology of quinolizidine alkaloids
3.2.4.1.1 Animal study data
The available animal studies generally examined extracts or feed with an unknown quinolizidine alkaloid profile, which does not allow conclusions to be drawn about the effects of individual alkaloids. Only a few studies were conducted with isolated quinolizidine alkaloids. These primarily investigated lupanine and sparteine, while one study also included other alkaloids such as albine and angustifoline.
The oral LD50 values in mice ranged between 60 and 220 mgshort formilligram/kgshort forkilogram for sparteine and between 159 and 410 mgshort formilligram/kgshort forkilogram for lupanine (Yovo et al. 1984; Aniszewski 2015). The LD50 for 13α-OH-lupanine is reported to be 189 mgshort formilligram/kgshort forkilogram. The potency of these compounds is therefore similar in terms of acute toxicity, with sparteine having the highest potency. In addition, there are species differences; for example, the LD50 values for lupanine range from 159 mgshort formilligram/kgshort forkilogram (mouse) to 1664 mgshort formilligram/kgshort forkilogram (rat) (Petterson et al. 1987; Aniszewski 2015). Typical symptoms of increased quinolizidine alkaloid intake are tremors, convulsions, cyanosis and tonic-clonic seizures.
In (sub)chronic studies in rats, no changes in food intake, body weight development, organ weights or macroscopic and microscopic organ changes were observed after long-term quinolizidine alkaloid intake compared to the control group (Schoeneberger et al. 1987). Findings regarding liver changes (decrease or increase in relative liver weights, no confirmation of the induction of liver foci observed in one study by the results of other studies) and haematological changes are inconsistent and in some cases contradictory. They are not considered a suitable basis for risk assessment (Ballester et al. 1982; Ballester et al. 1984; Butler et al. 1996; Robbins et al. 1996).
Most of the available data on the toxicity of quinolizidine alkaloids date from the period between 1980 and 2000. In the period specifically considered here, no studies providing new findings relevant to the assessment have been published since 2017. In particular, there is still a lack of toxicological data for risk assessment of quinolizidine alkaloids that have hardly been studied but are also found in food, such as angustifoline, multiflorine and albine. Accordingly, there is still a need for research into the varying potency of quinolizidine alkaloids which have hardly been studied to date. Furthermore, there is a lack of meaningful (sub)chronic studies on long-term effects and investigations into the combined effects of quinolizidine alkaloids.
3.2.4.1.2 Human data
Only very few meaningful studies investigating the adverse health effects of quinolizidine alkaloid intake in humans are available.
In 2019, the European Food Safety Authority (EFSAshort forEuropean Food Safety Authority) published a comprehensive opinion on the health risks to humans and animals posed by the presence of quinolizidine alkaloids in food and feed. The opinion focused on the quinolizidine alkaloids found in lupin species relevant to food and feed production in Europe (in particular L. albus, L. angustifolius, L. luteus and L. mutabilis Sweet). In its opinion, the EFSAshort forEuropean Food Safety Authority concluded that the data on the toxicity of the substances was insufficient at the time of the research (research period 1950–2017, additional research for “sparteine” in 2019) (EFSAshort forEuropean Food Safety Authority 2019). Based on the available data, the EFSAshort forEuropean Food Safety Authority was unable to determine a toxicological reference point for assessing the health risk to humans in the event of chronic exposure. To assess the health risk to humans in the event of acute exposure, the anticholinergic effects and the influence on the electrical conduction system of the heart were considered to be the most sensitive toxicological endpoint, and the lowest oral effect dose of 0.16 mgshort formilligram/kgshort forkilogram BW from human data for sparteine was used as the toxicological reference point for a margin of exposure (MOEshort forMargin of Exposure) consideration. The other quinolizidine alkaloids were assumed to have a comparable effect and potency to sparteine and a group assessment with dose additivity was performed for all the compounds. The acute toxicity data available for at least sparteine, lupanine and 13α-OH-lupanine, which indicate similar potency, make this approach appear reasonable given the limited data available. The dose of 0.16 mgshort formilligram/kgshort forkilogram BW was compared with the estimated acute exposure in humans, which was determined on the basis of the available data for specific scenarios. According to EFSA’s assessment, there are no health concerns at an MOEshort forMargin of Exposure >1.
In its report, the EFSAshort forEuropean Food Safety Authority emphasises that there is a lack of data on toxicokinetics and chronic toxicity, potential combination effects and toxic potencies of the relevant quinolizidine alkaloids (EFSAshort forEuropean Food Safety Authority 2019). The BfRshort forGerman Federal Institute for Risk Assessment also came to a similar conclusion in its previous opinion from 2017 (BfRshort forGerman Federal Institute for Risk Assessment 2017).
The case reports and documented cases of poisoning in humans published since 2017, which are specifically considered here, indicate that undesirable acute effects can occur in humans after consumption of products containing lupin seeds with a high alkaloid content, although the ingested alkaloid content is only documented in rare cases and there are therefore numerous uncertainties regarding the interpretation of the symptoms described. The symptoms range from non-specific effects, such as mild gastrointestinal complaints, to neurological disorders. The findings are generally consistent with the expected anticholinergic effects of quinolizidine alkaloids. In most cases, the symptoms improved within hours or a few days after consuming food containing lupin seeds. It can be assumed that children are more sensitive to the intake of quinolizidine alkaloids via food than adults. Even a very small number of seeds with a high alkaloid content can cause severe poisoning. However, there are no systematic studies on this subject. The data available since 2017 do not provide any new insights into the dose-response relationship or the potency of individual compounds.
3.2.4.1.3 Uncertainties
In the EU, the assessment of the health risk to humans in the event of acute exposure is based on human data for sparteine. The other quinolizidine alkaloids were assumed to have a comparable effect and potency to sparteine and a group assessment with dose additivity was carried out for all the compounds.
While similar effects such as tremors, tonic-clonic convulsions, cyanosis, collapse and death are generally described after short-term intake of high levels of quinolizidine alkaloids (Yovo et al. 1984; Petterson et al. 1987; Stobiecki et al. 1993), there are differences in receptor affinities at the nicotinic and muscarinic acetylcholine receptors, for example (Yovo et al. 1984; Schmeller et al. 1994). Studies on the long-term intake of high levels of quinolizidine alkaloids have mainly been conducted with lupin extracts, so no conclusions can be drawn here about the effects of individual quinolizidine alkaloids.
In addition, sparteine occurs in comparatively low quantities in most lupin species, with the exception of L. luteus. Content data show that lupanine and 13α-OH-lupanine predominate in the varieties used for food production. Other alkaloids found in the relevant product groups are angustifoline, sparteine, albine, multiflorine and isolupanine. It is still not possible to derive a dose-response relationship for the acute effects of the various quinolizidine alkaloids found in food after oral intake.
In its 2019 opinion, the EFSAshort forEuropean Food Safety Authority had already pointed out that there was also a lack of data on chronic toxicity and that it was therefore not possible to characterise the risk of chronic exposure to quinolizidine alkaloids via food (EFSAshort forEuropean Food Safety Authority 2019). This assessment remains valid after reviewing the current literature.
3.2.4.2 Assessment of the data available on quinolizidine alkaloid levels in food
In its 2019 opinion, the EFSAshort forEuropean Food Safety Authority assessed the data on exposure to quinolizidine alkaloids via food at the time of the research (consumption data from the EFSAshort forEuropean Food Safety Authority Comprehensive European Food Consumption Database, version 2018) as insufficient (EFSAshort forEuropean Food Safety Authority 2019).
Current data from the monitoring programmes of the Federal States in Germany and data from the published literature show that the levels of quinolizidine alkaloids are highest in foods in which lupin seeds make up a significant proportion, such as flours, meals and coffee substitutes. Here, levels of several hundred to thousand mgshort formilligram/kgshort forkilogram are reported in commercially available products. These levels would exceed the maximum level of 200 mgshort formilligram/kgshort forkilogram recommended by ANZFA (Australia New Zealand Food Authority; now Food Standards Australia New Zealand – FSANZ) (ANZFA 2001). Overall, however, the available data show large variations both between and within the different product groups.
Tests on raw seeds, some of which are bitter varieties, show levels of over 20,000 mgshort formilligram/kgshort forkilogram total alkaloids. Comparatively low total alkaloid levels of less than 50 mgshort formilligram/kgshort forkilogram were measured in other commercially available processed products that contain lupin seeds as an ingredient, such as vegetarian spreads or milk substitutes. Another possible source of exposure is animal products in which quinolizidine alkaloids have been transferred from feed to animal products such as meat and milk. The limited content data available from the milk monitoring programme (maximum <1.2 mgshort formilligram/kgshort forkilogram) and from published transfer studies for milk (95th percentile 8 mgshort formilligram/kgshort forkilogram) and muscle meat and liver samples (maximum <0.12 mgshort formilligram/kgshort forkilogram) showed very low total levels of quinolizidine alkaloids in the products, although the small number of samples and the high levels of variation within the product groups must be taken into account here. Due to the for the most part small number of samples within the individual relevant food categories, it is currently not possible to obtain a comprehensive picture of the individual sources of exposure.
In addition to the choice of variety, the different processing methods used in food production (debittering (see 3.2.1.1.3), heat treatment, etc.) can also have a significant influence on both the total content of quinolizidine alkaloids and the content of the individual alkaloids.
Furthermore, only limited data on consumption behaviour (consumption volumes and frequency) with regard to products containing lupin seeds are currently available not only for Germany but also for Europe. Therefore, there is still a lack of representative data on consumption patterns and comprehensive data on the levels of quinolizidine alkaloids in the various commercially available foods containing lupin seeds and consumed by consumers, which is necessary for a comprehensive exposure assessment of quinolizidine alkaloids from foods containing lupin seeds.
3.2.4.3 Evaluation of the data available on allergens in foods containing lupin seeds
Very low eliciting doses of a few 100 mgshort formilligram (provocation tests) or 3 mgshort formilligram or less for the occurrence of subjective symptoms and 300 mgshort formilligram or more of lupin flour for objective symptoms can be derived from publications evaluated by the BfRshort forGerman Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment 2011), and the publication by Peeters et al. (Peeters et al. 2007). In a study by Aguilera-Insunza et al. (Aguilera-Insunza et al. 2023), the minimum elicitation dose for symptoms of anaphylactic reactions was reported to be 1 g of lupin flour.
In the above-mentioned cross-reactivity study (Moneret-Vautrin et al. 1999), symptoms were observed after oral provocation at cumulative doses between 265 and 7,110 mgshort formilligram of lupin flour.
In the updated list of “Priority Food Allergens” in the Meeting Report of the Codex Alimentarius Group (FAO & WHO 2022), lupin is no longer listed as a priority allergen, but is only included in a list of allergens of regional significance. The reason for this is that the criteria of prevalence, potency and severity of effects are taken into account for inclusion on the priority list. With regard to lupin, the prevalence in unselected populations is assessed as undefined for each age group and each region evaluated. Based on provocation tests, an ED10 in the range of 10 to 100 mgshort formilligram protein was determined. Overall, there are only a few cases of anaphylactic reactions compared to prioritised allergens. The Codex Alimentarius Group’s Meeting Report states that, based on an evaluation of various studies, less than 20 % of people with peanut allergy react to lupin. However, concerns have been raised about the potential severity of lupin-induced reactions in people with peanut allergy (Shaw et al. 2008; Fiocchi et al. 2009; Mennini et al. 2016). Therefore, the problem is considered to be more significant for countries with a high prevalence of peanut allergies if consumption patterns were to change there.
Some authors refer to lupin as a “hidden allergen” (De las Marinas et al. 2007; Pouessel et al. 2024) or even a “hidden killer” (Campbell & Yates 2010) with the intention of drawing attention to the increased risk of anaphylaxis associated with lupin as a non-obvious allergen in processed flour for pasta or baked goods compared to easily recognisable whole peanuts, for example.
In summary, it can be said that since the last BfRshort forGerman Federal Institute for Risk Assessment Opinion on allergic reactions to lupin protein (BfRshort forGerman Federal Institute for Risk Assessment 2011), relevant specialist publications have appeared, particularly on prevalence in countries with a strong market presence of lupin flour products, such as France, on cross-reactivity and on the detection of various lupin allergens. In fact, about one in five people with peanut allergy have a cross-allergy to lupin, but primary sensitisation to lupin independently of peanut allergens has also been demonstrated in studies using combined, specific detection tests. Even though there are fewer reports of anaphylactic reactions in direct comparison to peanuts, this may be due to the (still) comparatively low market presence of unprocessed lupin seeds in particular. Peanuts, on the other hand, tend to be consumed unprocessed. Lupin seeds are often exposed to baking and other processing methods in the form of lupin flour in finished products and can lose their allergenic potential at high temperatures. Due to these different forms of consumption, prevalence and clinical reaction data are not directly comparable, especially in individual countries. However, the severity of reaction and the symptoms of lupin allergy are very similar to the reactions in people with peanut allergy.
3.3 Framework for action, recommended measures
Due to the insufficient data available to date, there are a number of uncertainties associated with the assessment of the health risks posed by lupin seeds in food. In the context of considerations to reduce these uncertainties, the following aspects are considered worthy of consideration:
(1) Collection of further data on the toxicity of quinolizidine alkaloids
The new data published since 2017 do not provide any new findings relevant to the assessment of the toxicity of quinolizidine alkaloids, meaning that the data available, particularly on the toxicity of quinolizidine alkaloids that have not yet been extensively studied but are also found in food (e.g. angustifoline, multiflorine and albine), remains insufficient for a comprehensive assessment.
With regard to acute toxicity, data on the potency of various quinolizidine alkaloids would be desirable. Findings from LD50 studies in mice and rats are available for the compounds lupanine, 13α-OH-lupanine and sparteine. For other quinolizidine alkaloids, only isolated data from in vitro studies on individual endpoints are available, but these are difficult to interpret due to the complex mechanisms of action (inhibition of muscarinic and nicotinic acetylcholine receptors, influence on ion channels, etc.).
Data on the (sub)chronic toxicity of quantitatively dominant quinolizidine alkaloids could be obtained by means of (sub)chronic studies on rodents, ideally using isolated pure substances. If these are not available in sufficient quantity and purity, feeding studies with lupin seeds are also conceivable. In this case, however, care should be taken to test several lupin species, each covering a characteristic quinolizidine alkaloid profile. The quinolizidine alkaloid profiles of the seeds examined should also be comprehensively characterised. The usefulness of in vitro findings on individual endpoints for risk assessment is probably low.
The toxicological data could also be used to derive scientifically based maximum levels for the presence of quinolizidine alkaloids in food in order to limit their occurrence in food.
(2) Collection of data on the consumption of foods containing lupins
A comprehensive exposure assessment requires representative data on consumption patterns and extensive data on the levels of quinolizidine alkaloids in commercially available foods containing lupin seeds.
For Germany and the European Union, only limited data on consumption behaviour (consumption volumes and frequency) for foods containing lupin seeds are currently available. These should be provided as part of a representative consumer survey and should include both short-term and long-term consumption.
(3) Collection of data on quinolizidine alkaloid levels in food
Due to the for the most part small number of samples within the individual relevant food categories, it is currently not possible to make statements on the individual sources of exposure. To improve the data situation, it is recommended that the quantity and type of lupin seeds or products made from them, such as lupin flour, used in ready-to-eat foods be collected and that lupin seeds, lupin flour and ready-to-eat products be tested for their quinolizidine alkaloid content. The focus should be on those quinolizidine alkaloids which predominate in quantity terms in the four lupin species used in the food sector. These include, in particular, lupanine, 13α-OH-lupanine, isolupanine, sparteine, albine, lupinine and angustifoline. The data could be collected, for example, as part of nationwide monitoring projects.
Such data could also provide information on the reduction or accumulation of quinolizidine alkaloids during food processing.
Data on the transfer of quinolizidine alkaloids from feed containing lupin seeds to animal-based food could also contribute to an improved exposure estimate.
(4) Further development of methods for quantifying a broad spectrum of quinolizidine alkaloids in food
In order to enable the quantification of a broad spectrum of quinolizidine alkaloids in food, reference standards of sufficient purity must be commercially available. To date, only the quinolizidine alkaloids currently available as standards have been considered. This results in an inconsistent picture with regard to the scope of the quinolizidine alkaloid spectrum considered and, where applicable, the total quinolizidine alkaloid levels calculated on this basis. In addition, there is the question of other quinolizidine alkaloids in lupins that have not yet been considered but may be toxicologically relevant. The availability of isotope-labelled standards could simplify analysis and increase its reliability.
In order to expand the data basis for risk assessment and to establish toxicologically justified maximum levels in the future, it is recommended that systematic monitoring programmes for the investigation of quinolizidine alkaloids in food be continued. This requires comparable methods for the determination of quinolizidine alkaloids. The validation of a harmonised method within the framework of a method validation study can make an important contribution here. In addition, it is necessary to expand the range of suitability tests available to verify the comparability of the methods used and to ensure the availability of certified reference materials.
(5) Measures to reduce quinolizidine alkaloid levels in food
The data summarised in the opinion show that cases of poisoning in consumers have been documented, particularly after consumption of highly contaminated products. From a toxicological point of view, maximum levels could be appropriate to limit the occurrence of quinolizidine alkaloids in food. These could initially be derived on the basis of technologically achievable levels.
Based on the available data, it is currently not possible to derive toxicologically justified maximum levels for the presence of quinolizidine alkaloids in food. In particular, there is a lack of data on the consumption of foods containing lupin seeds and on the levels of the various quinolizidine alkaloids in different products used as food.
The evaluated data also show that there have been no systematic studies to date on the quality of non-industrial debittering methods that are practicable for consumers as a basis for recommendations on debittering in private households.
(6) Investigations into the allergenic potential of certain lupin proteins
Due to the increasing use of lupin seeds, it can be assumed that the frequency of allergic reactions, some of which can be severe, may increase.
However, there is currently insufficient data on how different processing methods can be used to reduce the allergenic potential during the production of foods containing lupin seeds.
(7) Increased information for consumers about allergenic risks
It would be desirable to provide consumers with more information about the allergenic risks of lupin seeds and their presence in food. This includes, for example, information about possible cross-reactions in people with peanut and soy allergies.
4 References
AGES (Austrian Agency for Health and Food Safety) (2022). Quinolizidine alkaloids in products containing lupins – monitoring. Final report of priority action A-022-22. www.ages.at/en/human/focus/focus-actions/detail/quinolizidine-alkaloids-in-lupine-containing-products-monitoring
Agnew U., Dubensky L., Tortora L. (2020). Antimuscarinic Toxicity Due to Lupini Bean Ingestion. Clinical Toxicology 58: 1089.
Aguilera-Insunza R., Iturriaga C., Marinanco A., Venegas L., Aravena G., Perez-Mateluna G., Baptista-Dias N., Borzutzky A., Wandersleben T. (2023). High prevalence of lupin allergy among patients with peanut allergy: Identification of γ-conglutin as major allergen. Annals of Allergy, Asthma & Immunology 130: 225-232.
Aguilera J. M., Gerngross M. F., Lusas E. W. (1983). Aqueous processing of lupin seed. International Journal of Food Science and Technology 18: 327-333.
Aktories K., Förstermann U., Hofmann F. B., Starke K. (2009). General and Special Pharmacology and Toxicology. Founded by W. Forth, D. Henschler, W. Rummel, Elsevier GmbH, Munich.
Al-Abdouh A., Alrawashdeh H. M., Khalaf M. D., Alnawaiseh I. (2020). Anticholinergic Toxicity Associated with Lupine Seeds Ingestion - A Case Report. Research in Health Science 5: 22-26.
Alessandro L., Wibecan L., Cammarota A., Varela F. (2017). Pupillary Disorders in the Emergency Room: Lupinus mutabilis Intoxication. Journal of Clinical Toxicology 07
Alsakha A. and Eggleston W. (2023). Antimuscarinic Toxidrome Caused by Lupin Bean Ingestion. Journal of Medical Toxicology 19: 158.
Álvarez-Álvarez J, Guillamón E., Crespo J. F., Cuadrado C., Burbano C., Rodríguez J., Fernández C., Muzquiz M. (2005). Effects of Extrusion, Boiling, Autoclaving, and Microwave Heating on Lupine Allergenicity. Journal of Agricultural and Food Chemistry 53: 1294-1298.
Aniszewski Tadeusz (2015). Chapter 3 - Alkaloids in biology. In Alkaloids (Second Edition), Aniszewski T. (ed), pp 195-258. Elsevier, Boston.
Annicchiarico Pshort forphosphorus., Manunza Pshort forphosphorus., Arnoldi A., Boschin G. (2014). Quality of Lupinus albus L. (white lupin) seed: extent of genotypic and environmental effects. Journal of Agricultural and Food Chemistry 62: 6539-6545.
ANZFA (Australia New Zealand Food Authority) (2001). Lupin alkaloids in food - a toxicological review and risk assessment. Technical Report Series No 3
Ballester D., Yáñez E., García R., Erazo S., López F., Haardt E., Cornejo S., López A., Pokniak J., Chichester C. Oshort foroxygen. (1980). Chemical composition, nutritive value, and toxicological evaluation of two species of sweet Lupine (Lupinus albus and Lupinus luteus). Journal of Agricultural and Food Chemistry 28: 402-405.
Ballester D., Saitúa M. T., Brunser Oshort foroxygen, Egana J. I., Owen D. F., Yáñez E. (1982). Toxicological evaluation of sweet lupine. I. Study in rats fed for 9 months with Lupinus albus var. Multolupa. Revista Chilena de Nutricion 10: 177-179.
Ballester D. R., Brunser Oshort foroxygen., Saitua M. T., Egana J. I., Yanez E. Oshort foroxygen., Owen D. F. (1984). Safety evaluation of sweet lupine (Lupinus albus cv. Multolupa). II. Nine-month feeding and multigeneration study in rats. Food and Chemical Toxicology 22: 45-48.
Beyer B., Obrist D., Czarda Pshort forphosphorus., Puhringer K., Vymyslicky F., Siegmund B., D'Amico S., Cichna-Markl M. (2024). Influence of Roasting Temperature on the Detectability of Potentially Allergenic Lupin by SDS-PAGE, ELISAs, LC-MS/MS, and Real-Time PCR. Foods 13: 673.
BfRshort forGerman Federal Institute for Risk Assessment (German Federal Institute for Risk Assessment) (2011). Allergy caused by lupin protein in food. Updated opinion No. 039/2011 of the BfR dated 26 August 2011.
BfRshort forGerman Federal Institute for Risk Assessment (German Federal Institute for Risk Assessment) (2017). Risk assessment of alkaloid occurrence in lupin seeds. Opinion 003/2017 of the BfR dated 27 March 2017.
Blaschek W., Ebel S., Hackenthal E., Holzgrabe U., Keller K., Reichling J., Schulz V. (2006). HagerROM 2006. Hager's Handbook of Drugs and Medicinal Substances, Springer-Verlag Berlin Heidelberg.
Blaschek W., Ebel S., Hilgenfeldt U., Holzgrabe U., Reichling J., Schulz V., Barthlott W., Höltje H.-D. (2023). Hager's Encyclopaedia of Drugs and Medicines. plus.drugbase.de/de (accessed on 04.06.2024).
BLE (German Federal Office for Agriculture and Food) (2023). Market research on legumes (including soybeans) – Federal Information Centre for Agriculture (BZL). www.ble.de/SharedDocs/Downloads/DE/BZL/Daten-Berichte/OeleFette/JaehrlicheErgebnisse/2022_Bericht_Marktlage_Huelsenfruechte.pdf
Bleitgen R., Gross R., Gross U. (1979). Lupins – a contribution to food supply in the Andes – 5. Some observations on the traditional debittering of lupins in water. Journal of Nutrition Science 18: 104-111.
BMELshort forGerman Federal Ministry of Food and Agriculture (German Federal Ministry of Food and Agriculture) (2020). Field beans, peas & co. The protein plant strategy of the Federal Ministry of Food and Agriculture to promote legume cultivation in Germany.
BMELshort forGerman Federal Ministry of Food and Agriculture (German Federal Ministry of Food and Agriculture) (2023). BMELshort forGerman Federal Ministry of Food and Agriculture protein crop strategy. www.bmel.de/DE/themen/landwirtschaft/pflanzenbau/ackerbau/eiweisspflanzenstrategie.html (accessed on 12 June 2024)
Boschin G., Annicchiarico Pshort forphosphorus., Resta D., D'Agostina A., Arnoldi A. (2008). Quinolizidine alkaloids in seeds of lupin genotypes of different origins. Journal of Agricultural and Food Chemistry 56: 3657-3663.
Boschin G. and Resta D. (2013). Alkaloids derived from lysine: Quinolizidine (a focus on lupin alkaloids), Springer Berlin Heidelberg.
Boschin G., Tesio E., Arnoldi A. (2022). A field case of pig poisoning by accidental feed contamination by alkaloid-rich lupin seeds. Journal of Applied Animal Research 50: 725-731.
Brennecke S., Becker W. M., Lepp U., Jappe U. (2007). Anaphylactic reaction to lupine flour. JDDG: Journal of the German Dermatological Society 5: 774-776.
Bunsupa S., Yamazaki M., Saito K. (2012). Quinolizidine alkaloid biosynthesis: recent advances and future prospects. Frontiers in Plant Science 3: 239.
Butler W. H., Ford G. Pshort forphosphorus., Creasy D. M. (1996). A 90-day feeding study of lupin (Lupinus angustifolius) flour spiked with lupin alkaloids in the rat. Food and Chemical Toxicology 34: 531-536.
BVLshort forGerman Federal Office of Consumer Protection and Food Safety (German Federal Office of Consumer Protection and Food Safety) (2023). Food Safety Reports 2022: Monitoring. Joint report by the Federal Government and the Länder. www.bvl.bund.de/SharedDocs/Downloads/01_Lebensmittel/01_lm_mon_dokumente/01_Monitoring_Berichte/2022_lm_monitoring_bericht.pdf;jsessionid=965F2427AFF308C3653BDAD5F7269805.internet992.
BVLshort forGerman Federal Office of Consumer Protection and Food Safety (German Federal Office of Consumer Protection and Food Safety) (2024). BVLshort forGerman Federal Office of Consumer Protection and Food Safety Report – Reports on Food Safety: Monitoring 2023. Joint report by the Federal Government and the Länder
Camacho L., Sierra C., Marcus D., Guzman E., Campos R., von Baer D., Trugo L. (1991). Nutritional quality of lupine (Lupinus albus cv. Multolupa) as affected by lactic acid fermentation. International Journal of Food Microbiology 14: 277-286.
Campbell C. Pshort forphosphorus. and Yates D. H. (2010). Lupin allergy: a hidden killer at home, a menace at work; occupational disease due to lupin allergy. Clinical and Experimental Allergy 40: 1467-1472.
Carlier J., Guitton J., Romeuf L., Bevalot F., Boyer B., Fanton L., Gaillard Y. (2015). Screening approach by ultra-high performance liquid chromatography-tandem mass spectrometry for the blood quantification of thirty-four toxic principles of plant origin. Application to forensic toxicology. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences 975: 65-76.
Carvajal-Larenas F. E., Nout M. J. R., van Boekel M. A. J. S., Koziol M., Linnemann A. R. (2013). Modelling of the aqueous debittering process of Lupinus mutabilis Sweet. LWT - Food Science and Technology 53: 507-516.
Carvajal-Larenas F. E., Linnemann A. R., Nout M. J., Koziol M., van Boekel M. A. (2016). Lupinus mutabilis: Composition, Uses, Toxicology, and Debittering. Critical Reviews in Food Science and Nutrition 56: 1454-1487.
Cely-Veloza W., Quiroga D., Coy-Barrera E. (2022). Quinolizidine-Based Variations and Antifungal Activity of Eight Lupinus Species Grown under Greenhouse Conditions. Molecules 27: 305.
Chilomer K., Zaleska K., Ciesiołka D., Gulewicz Pshort forphosphorus., Frankiewicz A., Gulewicz K. (2011). Changes in the alkaloid, α-galactoside and protein fractions content during germination of different lupin species. Acta Societatis Botanicorum Poloniae 79: 11-20.
Chludil H. D., Vilarino M. del Pshort forphosphorus., Franco M. L., Leicach S. R. (2009). Changes in Lupinus albus and Lupinus angustifolius alkaloid profiles in response to mechanical damage. Journal of Agricultural and Food Chemistry 57: 6107-6113.
Christiansen J. L., Jørnsgård B., Buskov S., Olsen C. E. (1997). Effect of drought stress on content and composition of seed alkaloids in narrow-leafed lupin, Lupinus angustifolius L. European Journal of Agronomy 7: 307-314.
Chrubasik-Hausmann S. (2022). Besenginsterkraut: A promising medicinal plant for cardiovascular complaints. Thieme. Natural medicine! natuerlich.thieme.de/therapieverfahren/phytotherapie/detail/besenginsterkraut-vielversprechende-heilpflanze-bei-herz-kreislauf-beschwerden-249 (accessed on 19 June 2024).
Cortés-Avendaño Pshort forphosphorus., Tarvainen M., Suomela J. Pshort forphosphorus., Glorio-Paulet Pshort forphosphorus., Yang B., Repo-Carrasco-Valencia R. (2020). Profile and Content of Residual Alkaloids in Ten Ecotypes of Lupinus mutabilis Sweet after Aqueous Debittering Process. Plant Foods for Human Nutrition 75: 184-191.
Cousin M., Verdun S., Seynave M., Vilain A. C., Lansiaux A., Decoster A., Sauvage C. (2017). Phenotypical characterisation of peanut allergic children with differences in cross-allergy to tree nuts and other legumes. Paediatric Allergy and Immunology 28: 245-250.
Czarnecka E., Kolińska-Marzec A., Szadowska A. (1967). [Effect of certain lupanin alkaloids on post-aconitine arrhythmia of the isolated heart]. Acta Poloniae Pharmaceutica - Drug Research 24: 545-548.
Czerwenka C. and Dorn E. (2022). Development of a multi-method for quinolizidine alkaloids and its application to a variety of lupine-based food products. rafa2022.eu/pdf/Book%20of%20Abstracts%20RAFA%202022.pdf (accessed on 25 January 2024).
de Cortes Sánchez M., Altares Pshort forphosphorus., Pedrosa M. M., Burbano C., Cuadrado C., Goyoaga C., Muzquiz M., Jiménez-Martínez C., Dávila-Ortiz G. (2005). Alkaloid variation during germination in different lupin species. Food Chemistry 90: 347-355.
De las Marinas D., Cojocariu Z., Escudero R., Pardo N., Sanz M. L. (2007). Anaphylaxis induced by lupine as a hidden allergen. Journal of Investigational Allergology & Clinical Immunology 17: 283-284.
DGE (German Nutrition Society, Thuringia section) (2016). Short presentations at the 24th Nutrition Conference on the topic: "Renaissance of plant protein". 1-9. dge-th.de/fileadmin/user_upload/Abstract_24_EFT_alle.pdf.
Direction générale de la santé and Bureau VS 3 (1998). Opinion of 17 March 1998 of the French High Council for Public Health (Food and Nutrition Section) on the use of lupin flour in human food. Official Bulletin No. 98/27.
EFSAshort forEuropean Food Safety Authority (European Food Safety Authority: Panel on Contaminants in the Food Chain (CONTAM)) (2019). Scientific opinion on the risks for animal and human health related to the presence of quinolizidine alkaloids in feed and food, in particular in lupins and lupin-derived products. EFSA Journal 17(11): 5860.
EFSAshort forEuropean Food Safety Authority (European Food Safety Authority: Scientific Committee) (2012). Guidance on selected default values to be used by the EFSAshort forEuropean Food Safety Authority Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 10(3): 2579.
Eichelbaum M., Spannbrucker N., Steincke B., Dengler H. J. (1979). Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. European Journal of Clinical Pharmacology 16: 183-187.
Engel A. M., Klevenhusen F., Moenning J. L., Numata J., Fischer-Tenhagen C., Sachse B., Schafer B., Fry H., Kappenstein Oshort foroxygen., Pieper R. (2022). Investigations on the transfer of quinolizidine alkaloids from Lupinus angustifolius into the milk of dairy cows. Journal of Agricultural and Food Chemistry 70: 11749-11758.
Erbas M. (2010). The Effects of Different Debittering Methods on the Production of Lupin Bean Snack from Bitter Lupinus Albus L. Seeds. Journal of Food Quality 33: 742-757.
Ertas N. and Bilgicli N. (2014). Effect of different debittering processes on mineral and phytic acid content of lupin (Lupinus albus L.) seeds. Journal of Food Science and Technology 51: 3348-3354.
Esparza C. L., Laencina L. Pshort forphosphorus., Naya L. B., Torrijos M. R., Brito M. Oshort foroxygen., Colás M. V. (2021). Poisoning from cooking water of lupins or lupin beans. Revista Sanitaria de Investigación 2: 83.
EU Reference Laboratory for mycotoxins & plant toxins in food and feed (2022). Determination of quinolizidine alkaloids in lupin seeds, food products and feed by LC-MS/MS. www.wur.nl/en/research-results/research-institutes/food-safety-research/reference-laboratory/european-union-reference-laboratory/eurl-mycotoxins-plant-toxins/library-eurl-mp.htm (accessed on 29 January 2024).
Eugelio F., Palmieri S., Fanti F., Messuri L., Pepe A., Compagnone D., Sergi M. (2023). Development of an HPLC-MS/MS Method for the Determination of Alkaloids in Lupins. Molecules 28: 1531.
European Commission (2024). Notification 2024.0777. Quinolizidine alkaloids (lupanine and sparteine) in lupine seeds from Lebanon. RASFF Window webgate.ec.europa.eu/rasff-window/screen/notification/661163 (accessed on 12 June 2024).
FAO (Food and Agriculture Organisation of the United Nations) and WHO (World Health Organisation) (2022). Risk Assessment of Food Allergens. Part 1 – Review and validation of Codex Alimentarius priority allergen list through risk assessment. Food Safety and Quality Series 14
FiBL (Research Institute of Organic Agriculture) (2024). Alkaloid analysis in lupins – Prerequisite for food production. Fact sheet No. 1763: 1-8.
Fiocchi A., Sarratud Pshort forphosphorus., Terracciano L., Vacca E., Bernardini R., Fuggetta D., Ballabio C., Duranti M., Magni C., Restani Pshort forphosphorus. (2009). Assessment of the tolerance to lupine-enriched pasta in peanut-allergic children. Clinical & Experimental Allergy 39: 1045-1051.
Flores-Pamo A. E., Pisano E., Carreazo N. Y. (2018). Anticholinergic toxicity in a one-year-old male following ingestion of lupinus mutabilis seeds: Case report. Sao Paulo Medical Journal 136: 591-593.
Frick K. M., Kamphuis L. G., Siddique K. H. M., Singh K. B., Foley R. C. (2017). Quinolizidine alkaloid biosynthesis in lupins and prospects for grain quality improvement. Frontiers in Plant Science 8: 87.
Frick K. M., Lorensen M. D. B. B., Esteban E., Pasha A., Schulz A., Provart N. J., Janfelt C., Nour-Eldin H. H., Geu-Flores F. (2023). The aerial epidermis is a major site of quinolizidine alkaloid biosynthesis in narrow-leafed lupin. bioRxiv: DOI: 10.1101/2023.1103.1120.532575.
FSA (Food Standards Agency: Advisory Committee on Novel Foods and Processes (ACNFP)) (1996). Annual Report. Appendix IX. ACNFP report on seeds from the narrow-leaved lupin (Lupinus angustifolius).
Fudiyansyah N., Petterson D. S., Bell R. R., Fairbrother A. H. (1995). A nutritional, chemical and sensory evaluation of lupin (L. angustifolius) tempe. International Journal of Food Science and Technology 30: 297-305.
Gayraud J., Mairesse M., Fontaine J. F., Thillay A., Leduc V., Rancé F., Parisot L., Moneret-Vautrin D. A. (2009). The prevalence of sensitisation to lupin flour in France and Belgium: a prospective study in 5,366 patients, by the Allergy Vigilance Network. European Annals of Allergy and Clinical Immunology 41: 17-22.
Gessner Oshort foroxygen. and Orzechowski G. (1974). Poisonous and medicinal plants of Central Europe, Universitätsverlag Winter, Heidelberg.
Golebiewski W. M. and Spenser I. D. (1988). Biosynthesis of the lupine alkaloids. II. Sparteine and lupanine. Canadian Journal of Chemistry 66: 1734-1748.
Grant G., Dorward Pshort forphosphorus. M., Pusztai A. (1993). Pancreatic enlargement is evident in rats fed diets containing raw soybeans (Glycine max) or cowpeas (Vigna unguiculata) for 800 days but not in those fed diets based on kidney beans (Phaseolus vulgaris) or lupinseed (Lupinus angustifolius). Journal of Nutrition 123: 2207-2215.
Grant G., Dorward Pshort forphosphorus. M., Buchan W. C., Armour J. C., Pusztai A. (1995). Consumption of diets containing raw soya beans (Glycine max), kidney beans (Phaseolus vulgaris), cowpeas (Vigna unguiculata) or lupin seeds (Lupinus angustifolius) by rats for up to 700 days: effects on body composition and organ weights. British Journal of Nutrition 73: 17-29.
Green B. T., Lee S. T., Welch K. D., Gardner D. R., Stegelmeier B. L., Davis T. Z. (2015). The serum concentrations of lupine alkaloids in orally-dosed Holstein cattle. Research in Veterinary Science 100: 239-244.
Gresta F., Abbate V., Avola G., Magazzu G., Chiofalo B. (2010). Lupin Seed for the Crop-Livestock Food Chain. Italian Journal of Agronomy 5: 333-340.
Griffiths M. R., Strobel B. W., Hama J. R., Cedergreen N. (2021). Toxicity and risk of plant-produced alkaloids to Daphnia magna. Environmental Sciences Europe 33
Grundmann S. A., Mertens M., Hungeling S., Brehler R. (2008). Lupin flour – an allergen gains importance. Current Dermatology 34: 270-273.
Haddad J., Muzquiz M., Allaf K. (2006). Treatment of lupin seed using the instantaneous controlled pressure drop technology to reduce alkaloid content. Food Science and Technology International 12: 365-370.
Hatzold T., Elmadfa I., Gross R., Wink M., Hartmann T., Witte L. (1983). Quinolizidine alkaloids in seeds of Lupinus mutabilis. Journal of Agricultural and Food Chemistry 31: 934-938.
Hefle S. L., Lemanske R. F., Jr., Bush R. K. (1994). Adverse reaction to lupine-fortified pasta. Journal of Allergy and Clinical Immunology 94: 167-172.
Hondelmann W. (1984). The lupin - ancient and modern crop plant. Theoretical and Applied Genetics 68: 1-9.
Hwang I. M., Lee H. W., Lee H. M., Yang J. S., Seo H. Y., Chung Y. J., Kim S. H. (2020). Rapid and Simultaneous Quantification of Five Quinolizidine Alkaloids in Lupinus angustifolius L. and Its Processed Foods by UPLC–MS/MS. ACS Omega 5: 20825-20830.
Poison Control Centre NRW (2024). Broom (Cytisus [Sarothamnus] scoparius). gizbonn.de/giftzentrale-bonn/pflanzen/besenginster (accessed on 19 June 2024).
Jansen G., Jürgens H. U., Ordon F. (2009). Effects of temperature on the alkaloid content of seeds of Lupinus angustifolius cultivars. Journal of Agronomy and Crop Science 195: 172-177.
Jappe U. and Vieths S. (2010). Lupine, a source of new as well as hidden food allergens. Molecular Nutrition and Food Research 54: 113-126.
Jecsai J., Szelenyi-Galantai M., Juhasz B. (1986). Antinutritive effect of different lupin (Lupinus) species on the protein metabolism of rats. Acta Veterinaria Hungarica 34: 19-27.
Jiménez-Martínez C., Hernández-Sánchez H., Dávila-Ortiz G. (2007). Diminution of quinolizidine alkaloids, oligosaccharides and phenolic compounds from two species of Lupinus and soybean seeds by the effect of Rhizopus oligosporus. Journal of the Science of Food and Agriculture 87: 1315-1322.
Kamel K. A., Święcicki W., Kaczmarek Z., Barzyk Pshort forphosphorus. (2015). Quantitative and qualitative content of alkaloids in seeds of a narrow-leafed lupin (Lupinus angustifolius L.) collection. Genetic Resources and Crop Evolution 63: 711-719.
Karlsson E. M. and Peter H. W. (1978). Determination of alkaloids from Lupinus polyphyllus by quantitative thin-layer chromatography. Journal of Chromatography A 155: 218-222.
Keuth Oshort foroxygen., Humpf H. U., Fürst Pshort forphosphorus. (2023). Quinolizidine alkaloids in lupine flour and lupine products from the German retail market and risk assessment of the results regarding human health. Food Additives & Contaminants: Part A 40: 667-674.
Khan M. K., Karnpanit W., Nasar-Abbas S. M., Huma Z., Jayasena V. (2015). Phytochemical composition and bioactivities of lupin: a review. International Journal of Food Science and Technology 50: 2004-2012.
Khedr T., Juhász A., Singh K. B., Foley R., Nye-Wood M. G., Colgrave M. L. (2023). Development and validation of a rapid and sensitive LC-MS/MS approach for alkaloid testing in different Lupinus species. Journal of Food Composition and Analysis 121: 105391.
Kreuzer H. and Lüth U. (1959). Investigations into the effect of sparteine on the venous system. German Medical Weekly 84: 941-944.
Lagrange E., Vernoux J.-Pshort forphosphorus., Chambon C., Camu W., Spencer Pshort forphosphorus. S. (2024). Cramp–Fasciculation Syndrome Associated with Natural and Added Chemicals in Popular Food Items. Foods 13: 2257.
Lahoud C., Hanna N. G., Jalkh A., Azar G. (2021). Acute Bilateral Fixed Mydriasis Caused by Lupini Bean Intoxication. Wilderness and Environmental Medicine 32: 217-220.
Leduc V., Moneret-Vautrin D. A., Guérin L. (2002). [Allergenicity of lupin flour]. Allergie et Immunologie 34: 213-217.
Lee H. W., Hwang I. M., Lee H. M., Yang J. S., Park E. J., Choi J. W., Seo H. Y., Kim S. H. (2020). Validation and Determination of Quinolizidine Alkaloids (QAs) in Lupin Products by Gas Chromatography with Flame Ionisation Detection (GC-FID). Analytical Letters 53: 606-613.
Lee S. T., Stonecipher C. A., Dos Santos F. C., Pfister J. A., Welch K. D., Cook D., Green B. T., Gardner D. R., Panter K. E. (2019). An Evaluation of Hair, Oral Fluid, Earwax, and Nasal Mucus as Noninvasive Specimens to Determine Livestock Exposure to Teratogenic Lupine Species. Journal of Agricultural and Food Chemistry 67: 43-49.
Lishort forlithium K., van Wijk X. M. R., Hayashi S., Lynch K. L., Wu A. H. B., Smollin C. G. (2017). Anticholinergic toxicity associated with ingestion of water containing lupini bean extract. Clinical Toxicology 55: 687-688.
Ligon E. W. (1941). The action of lupine alkaloids on the motility of the isolated rabbit uterus. Journal of Pharmacology and Experimental Therapeutics 73: 151-158.
Lowen R. J., Alam F. K., Edgar J. A. (1995). Lupin bean toxicity. Medical Journal of Australia 162: 256-257.
Magalhães S. C. Q., Fernandes F., Cabrita A. R. J., Fonseca A. J. M., Valentão Pshort forphosphorus., Andrade Pshort forphosphorus. B. (2017). Alkaloids in the valorisation of European Lupinus spp. seeds crop. Industrial Crops and Products 95: 286-295.
Mancinotti D., Frick K. M., Geu-Flores F. (2022). Biosynthesis of quinolizidine alkaloids in lupins: mechanistic considerations and prospects for pathway elucidation. Natural Product Reports 39: 1423-1437.
Matheu V., de Barrio M., Sierra Z., Gracia-Bara M. T., Tornero Pshort forphosphorus., Baeza M. L. (1999). Lupine-induced anaphylaxis. Annals of Allergy, Asthma & Immunology 83: 406-408.
Mennini M., Dahdah L., Mazzina Oshort foroxygen., Fiocchi A. (2016). Lupin and Other Potentially Cross-Reactive Allergens in Peanut Allergy. Current Allergy and Asthma Reports 16: 84.
Mol H. G. J., Van Dam R. C. J., Zomer Pshort forphosphorus., Mulder Pshort forphosphorus. Pshort forphosphorus. J. (2011). Screening of plant toxins in food, feed and botanicals using full-scan high-resolution (Orbitrap) mass spectrometry. Food Additives & Contaminants: Part A 28: 1405-1423.
Moneret-Vautrin D. A., Guérin L., Kanny G., Flabbee J., Frémont S., Morisset M. (1999). Cross-allergenicity of peanut and lupine: The risk of lupine allergy in patients allergic to peanuts. Journal of Allergy and Clinical Immunology 104: 883-888.
Moneret-Vautrin D. A., Kanny G., Morisset M., Rancé F., Fardeau M. F., Beaudouin E. (2004). Severe food anaphylaxis: 107 cases registered in 2002 by the Allergy Vigilance Network. European Annals of Allergy and Clinical Immunology 36: 46-51.
Muffly M., Perlin A., Rodriguez M., Gebbia J., Gans M., Kleiner G. (2022). Lupine allergy cross-reactivity with Fabaceae family. Journal of Allergy and Clinical Immunology149: AB115.
Muller T., Luc A., Adam T., Jarlot-Chevaux S., Dumond Pshort forphosphorus., Schweitzer C., Codreanu-Morel F., Divaret-Chauveau A. (2022). Relevance of sensitisation to legumes in peanut-allergic children. Paediatric Allergy and Immunology 33: e13846.
Muzquiz M., Cuadrado C., Ayet G., Delacuadra C., Burbano C., Osagie A. (1994). Variation of alkaloid components of lupin seeds in 49 genotypes of Lupinus albus L. from different countries and locations. Journal of Agricultural and Food Chemistry 42: 1447-1450.
Namdar D., Mulder Pshort forphosphorus. Pshort forphosphorus. J., Ben-Simchon E., Hacham Y., Basheer L., Cohen Oshort foroxygen., Sternberg M., Shelef Oshort foroxygen. (2024). New analytical approach to quinolizidine alkaloids and their assumed biosynthesis pathways in lupin seeds. Toxins 16: 163.
Newton B. W., Benson R. C., McCorriston C. C. (1966). Sparteine sulphate: a potent, capricious oxytocic. American Journal of Obstetrics and Gynaecology 94: 234-241.
Ortega-David E. and Rodriguez-Stouvenel A. (2013). Degradation of quinolizidine alkaloids of lupin by Rhizopus oligosporus. Applied Microbiology and Biotechnology 97: 4799-4810.
Ortiz J. G. F. and Mukherjee K. D. (1982). Extraction of Alkaloids and Oil from Bitter Lupin Seed. Journal of the American Oil Chemists Society 59: 241-244.
Otterbach S. L., Yang T., Kato L., Janfelt C., Geu-Flores F. (2019). Quinolizidine alkaloids are transported to seeds of bitter narrow-leafed lupin. Journal of Experimental Botany 70: 5799-5808.
Ozkaya Pshort forphosphorus.Y., Ari H. F., Turanli E. E., Koc G., Karapinar B. (2021). Severe lupin bean intoxication: an anticholinergic toxidrome. Paediatric Emergency Medicine Journal 8: 108-111.
Peeters K. A., Nordlee J. A., Penninks A. H., Chen L., Goodman R. E., Bruijnzeel-Koomen C. A., Hefle S. L., Taylor S. L., Knulst A. C. (2007). Lupine allergy: Not simply cross-reactivity with peanut or soy. Journal of Allergy and Clinical Immunology 120: 647-653.
Petterson D. S., Ellis Z. L., Harris D. J., Spadek Z. E. (1987). Acute toxicity of the major alkaloids of cultivated Lupinus angustifolius seeds to rats. Journal of Applied Toxicology 7: 51-53.
Petterson D. S., Greirson B. N., Allen D. G., Harris D. J., Power B. M., Dusci L. J., Ilett K. F. (1994). Disposition of lupanine and 13-hydroxylupanine in man. Xenobiotica 24: 933-941.
Petterson D. S. (1998). Composition and food uses of lupins. In Lupins as Crop Plants: Biology, Production and Utilisation Gladstones J. S., Atkins C. A., Hamblin J. (eds), pp 353-383. CAB International, Wallingford, Oxon, UK.
Pilegaard K. and Gry J. (2008). Alkaloids in edible lupin seeds. A toxicological review and recommendations. TemaNord 605: 1-71.
Pouessel G., Sabouraud-Leclerc D., Beaumont Pshort forphosphorus., Divaret-Chauveau A., Bradatan E., Dumond Pshort forphosphorus., Karaca Y., Renaudin J. M., Metz-Favre C., Delalande D., Correard A. K., Tscheiller S., Van der Brempt X. (2024). Lupin, a potential "hidden" food anaphylaxis allergen: An alert from the Allergy-Vigilance Network®. Allergy 79: 2267-2270.
Priddis C. R. (1983). Capillary gas chromatography of lupin alkaloids. Journal of Chromatography A 261: 95-101.
Przybył A. K. and Kubicki M. (2011). Simple and highly efficient preparation and characterisation of (−)-lupanine and (+)-sparteine. Tetrahedron 67: 7787-7793.
Radcliffe M., Scadding G., Brown H. M. (2005). Lupin flour anaphylaxis. The Lancet 365: 1360.
Rahman M. H. (2000). The nutritional toxicity of sweet lupin (Lupinus angustifolius) seed proteins. Journal of the Science of Food and Agriculture 80: 72-78.
Raschack M. (1974). [Actions of sparteine and sparteine derivatives on the heart and circulation]. Arzneimittelforschung 24: 753-759.
Reinhard H., Rupp H., Sager F., Streule M., Zoller Oshort foroxygen. (2006). Quinolizidine alkaloids and phomopsins in lupin seeds and lupin-containing food. Journal of Chromatography A 1112: 353-360.
Reis A. M., Fernandes N. Pshort forphosphorus., Marques S. L., Paes M. J., Sousa S., Carvalho F., Conde T., Trindade M. (2007). Lupin sensitisation in a population of 1,160 subjects. Allergologia et Immunopathologia 35: 162-163.
Resta D., Boschin G., D'Agostina A., Arnoldi A. (2008). Evaluation of total quinolizidine alkaloids content in lupin flours, lupin-based ingredients, and foods. Molecular Nutrition and Food Research 52: 490-495.
Robbins M. C., Petterson D. S., Brantom Pshort forphosphorus. G. (1996). A 90-day feeding study of the alkaloids of Lupinus angustifolius in the rat. Food and Chemical Toxicology 34: 679-686.
Rodes-Bachs C. and Van der Fels-Klerx H. J. (2023). Impact of environmental factors on the presence of quinolizidine alkaloids in lupins: a review. Food Additives & Contaminants: Part A 40: 757-769.
Romeo F. V., Fabroni S., Ballistreri G., Muccilli S., Spina A., Rapisarda Pshort forphosphorus. (2018). Characterisation and antimicrobial activity of alkaloid extracts from seeds of different genotypes of Lupinus spp. Sustainability (Switzerland) 10: 788.
Ruiz Jr. L. Pshort forphosphorus. (1977). A rapid screening test for lupin alkaloids. New Zealand Journal of Agricultural Research 20: 51-52.
Ruiz Jr. L. Pshort forphosphorus., White S. F., Hove E. L. (1977). The alkaloid content of sweet lupin seed used in feeding trials on pigs and rats. Animal Feed Science and Technology 2: 59-66.
Santana F. C. and Empis J. (2001). Bacterial removal of quinolizidine alkaloids from Lupinus albus flours. European Food Research and Technology 212: 217-224.
Santiago Quiles M. R. , Oquendo-Jimenez I., Herreno-Saenz D., Antoun M. D. (2010). Genotoxicity of Alkaloid-Rich Extract from Lupinus termis Seeds. Pharmaceutical Crops: 18-23.
Schmeller T., Sauerwein M., Sporer F., Wink M., Muller W. E. (1994). Binding of quinolizidine alkaloids to nicotinic and muscarinic acetylcholine receptors. Journal of Natural Products 57: 1316-1319.
Schmidlin-Mészáros J. (1973). Food poisoning caused by lupin beans. Communications from the field of food testing and hygiene 64: 194–205.
Schmitt C., Torrents R., Domange B., De Haro L., Simon N. (2019). Anticholinergic toxicity associated with lupini beans in Europe: two case reports. Clinical Toxicology 57: 522.
Schoeneberger H., Morón S., Gross R. (1987). Safety evaluation of water debittered Andean lupins (Lupinus mutabilis): 12-week rat feeding study. Plant Foods for Human Nutrition 37: 169-182.
Schomerus M., Eichelbaum F. M., Dengler H. J. (1978). Pharmacokinetics of sparteine and verapamil. Schattauer, Stuttgart.
Schreiber U., Schulte M., Khlopushina A., Esselen M. (2025). Hazard Identification of Food-Relevant Lupine Alkaloids Focusing on In Vitro Genotoxicity and Mutagenicity. ACS Food Science and Technology
Schryvers S., Arinzechukwu C., Miserez B., Eeckhout M., Jacxsens L. (2023). The fate of quinolizidine alkaloids during the processing of lupins (Lupinus spp.) for human consumption. Food Chemistry 429: 136847.
Schryvers S., Jacxsens L., Croubels S., Vonck S., Miserez B., Van De Steene J., Necchi Rohers G., Eeckhout M. (2024a). Quinolizidine alkaloids and phomopsin A in animal feed containing lupins: co-occurrence and carry-over into veal products. Food Additives and Contaminants: Part A 41: 885-899.
Schryvers S., Jacxsens L., Croubels S., Vonck S., Miserez B., Van De Steene J., Necchi Rohers G., Eeckhout M. (2024b). Quinolizidine alkaloids and phomopsin A in animal feed containing lupins: co-occurrence and carry-over into veal products. Food Additives & Contaminants: Part A: 1-15.
Shaw J., Roberts G., Grimshaw K., White S., Hourihane J. (2008). Short communication: Lupin allergy in peanut-allergic children and teenagers. Allergy 63: 370-373.
Silva D., Parreira S., Antunes A. Pshort forphosphorus., Valadas A. F. (2020). Lupin bean intoxication: an odd case of dysautonomic symptoms. European Journal of Neurology 27: 749.
Smith R. A. (1987). Potential edible lupine poisonings in humans. Veterinary and Human Toxicology 29: 444-445.
Späth Gudrun (1982). Poisonings and acute drug overdoses: mechanism of action, emergency measures and intensive care. Vol. 2. Completely revised and expanded edition, De Gruyter, Berlin, Boston.
Stobiecki M., Blaszczyk B., Kowalczyk-Bronisz S. H., Gulewicz K. (1993). The toxicity of seed extracts and their fractions from Lupinus angustifolius L. and Lupinus albus L. Journal of Applied Toxicology 13: 347-352.
Thies Pshort forphosphorus. W. (1986). Spartium and sparteine. From broom to antiarrhythmic. Pharmacy in our time 15: 172-176.
Tirdilova I., Vollmannova A., Ceryova S., Obtulovic Pshort forphosphorus., Arvay J., Zetochova E. (2022). Impact of 3-Year Period as a Factor on the Content of Biologically Valuable Substances in Seeds of White Lupin. Plants (Basel) 11: 2087.
Torres Tello F., Nagata A., Dreifuss Spiegel W. (1980). [Methods of eliminating alkaloids from the seeds of Lupinus mutabilis Sweet]. Latin American Archives of Nutrition 30: 200-209.
Trautmann A. and Kleine-Tebbe J. (2022). Allergology in Clinic and Practice – Allergens – Diagnostics – Therapy. Vol. 4., completely revised edition, Thieme, Stuttgart.
Vanerková D., Marková L., Portychová L., Horna A., Jelınek M. (2014). Methodology for the safety assessment of lupin in terms of alkaloids content. In 14th International Nutrition & Diagnostics Conference; INDC, pp 7-82.
Villacrés E., Álvarez J., Rosell C. (2020). Effects of two debittering processes on the alkaloid content and quality characteristics of lupin (Lupinus mutabilis Sweet). Journal of the Science of Food and Agriculture 100: 2166-2175.
Vishnyakova M. A., Salikova A. V., Shelenga T. V., Egorova G. Pshort forphosphorus., Novikova L. Y. (2023). Alkaloid content variability in the seeds of narrow-leafed lupine accessions from the VIR collection under the conditions of the Russian Northwest. Vavilovskii Zhurnal Genetiki i Selektsii 27: 119-128.
Wink M. and Hartmann T. (1981). Activation of chloroplast-localised enzymes of quinolizidine alkaloid biosynthesis by reduced thioredoxin. Plant Cell Reports 1: 6-9.
Wink M., Witte L., Hartmann T., Theuring C., Volz V. (1983). Accumulation of Quinolizidine Alkaloids in Plants and Cell Suspension Cultures: Genera Lupinus, Cytisus, Baptisia, Genista, Laburnum, and Sophora. Planta Medica 48: 253-257.
Wink M., Meissner C., Witte L. (1995). Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry 38: 139-153.
Wink M. (2019). Quinolizidine and Pyrrolizidine Alkaloid Chemical Ecology – a Mini-Review on Their Similarities and Differences. Journal of Chemical Ecology 45: 109-115.
Wittenburg H. and Nehring K. (1965). Investigations into the effect of pure lupine alkaloids on the animal organism. The effect of lupanine on rats. Die Pharmazie 20: 156-158.
Yovo K., Huguet F., Pothier J., Durand M., Breteau M., Narcisse G. (1984). Comparative pharmacological study of sparteine and its ketonic derivative lupanine from seeds of Lupinus albus. Planta Medica 50: 420-424.
External Link:[1] The poisoning severity score (PSS) classifies health problems caused by poisoning into the following severity levels: 0 = asymptomatic; 1 = minor, 2 = moderate and 3 = severe symptoms, or: “unassessable”.
External Link:[2] Mintel GNPD - Global New Products Database. © 2023 Mintel Group Ltd, 11 Pilgrim Street, London, UK EC4V
5 Appendix
Table A1: GIZ survey on lupins (lupin products) 2016–2021 – Summary exposure data from four poison information centres (GIZ).
| GIZ | Year | All exposures | With plant parts | With food | Severity (Poisoning Severity Score, PSS) | GIZ note | |||||||
Total expos. | of which oral | Total plants | Plants of which oral | Total food | Food, of which oral | Asymptomatic | Mild | Moderate | Severe | Unass-essable | |||
| GIZ 1 | 2016 | 2 | 2 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | |
| GIZ 1 | 2017 | 7 | 6 | 7 | 6 | 0 | 0 | 5 | 1 | 0 | 0 | 1 | |
| GIZ 1 | 2018 | 13 | 11 | 12 | 10 | 1 | 1 | 11 | 2 | 0 | 0 | 0 | |
| GIZ 1 | 2019 | 14 | 12 | 13 | 12 | 1 | 1 | 10 | 3 | 1 | 0 | 0 | |
| GIZ 1 | 2020 | 13 | 13 | 12 | 12 | 1 | 1 | 10 | 2 | 1 | 0 | 0 | |
| GIZ 1 | 2021 | 10 | 9 | 10 | 9 | 0 | 0 | 8 | 2 | 0 | 0 | 0 | |
| GIZ 1 | Total | 59 | 53 | 56 | 51 | 3 | 3 | 45 | 11 | 2 | 0 | 1 | |
| GIZ 2 | 2016 | 4 | 4 | 4 | 4 | 4 | |||||||
| GIZ 2 | 2017 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | |||||
| GIZ 2 | 2018 | 4 | 4 | 3 | 3 | 1 | 1 | 3 | 1 | ||||
| GIZ 2 | 2019 | 4 | 2 | 4 | 2 | 2 | 1 | 1 | |||||
| GIZ 2 | 2020 | 7 | 7 | 7 | 7 | 6 | 1 | ||||||
| GIZ 2 | 2021 | 11 | 10 | 8 | 7 | 3 | 3 | 8 | 3 | ||||
| GIZ 2 | Total | 31 | 28 | 26 | 23 | 5 | 5 | 22 | 6 | 2 | 0 | 1 | Note: Cases are not tracked, therefore the maximum severity is indicated. |
| GIZ 3 | 2016 | 0 | 0 | 0 | |||||||||
| GIZ 3 | 2017 | 2 | 2 | 2 | 1 | 1 | 1 | Not oral: inhalation of pollen suspected, but connection with symptoms considered unlikely. | |||||
| GIZ 3 | 2018 | 7 | 6 | 6 | 6 | 1 | 1 | 6 | 1 | ||||
| GIZ 3 | 2019 | 4 | 4 | 4 | 4 | 4 | |||||||
| GIZ 3 | 2020 | 8 | 8 | 7 | 7 | 1 | 1 | 6 | 2 | ||||
| GIZ 3 | 2021 | 5 | 5 | 5 | 5 | 2 | 2 | 5 | |||||
| GIZ 3 | Total | 26 | 25 | 24 | 23 | 4 | 4 | 22 | 4 | 0 | 0 | 0 | N.B.: food product names are not available, only the entry that they were sweet lupins. |
| GIZ 4 | 2016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| GIZ 4 | 2017 | 2 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |||
| GIZ 4 | 2018 | 7 | 7 | 2 | 2 | 0 | 5 | 1 | 0 | 0 | 1 | ||
| GIZ 4 | 2019 | 9 | 7 | 0 | 0 | 7 | 2 | 0 | 0 | 0 | |||
| GIZ 4 | 2020 | 14 | 11 | 6 | 6 | 0 | 10 | 3 | 1 | 0 | 0 | ||
| GIZ 4 | 2021 | 12 | 8 | 3 | 2 | 0 | 11 | 1 | 0 | 0 | 0 | ||
| GIZ 4 | Total | 44 | 35 | 11 | 10 | 0 | 0 | 35 | 7 | 1 | 0 | 1 | |
| Total | 160 | 141 | 117 | 107 | 12 | 12 | 124 | 28 | 5 | 0 | 3 | ||
| PSS in % | 77.5 % | 17.5 % | 3.1 % | 0.0 % | 1.9 % | ||||||||
Further information on the BfRshort forGerman Federal Institute for Risk Assessment website on this topic
- FAQ: Lupins, insects or lab-grown meat: what is the current state of health risk assessment for alternative protein sources? Go to FAQ
- Press release: Lupin seeds: Health impairments possible with bitter taste Go to press release
- Food allergies topic page Go to page
