Psychoactive effects to be expected following consumption of products containing hexahydrocannabinol (HHC)
Changes to the version from 5 October 2023: Introduction of the new BfRshort forGerman Federal Institute for Risk Assessment risk profile, addition of new data, update regarding the legal classification of HHC.
What it's about:
- Hexahydrocannabinol (HHC) belongs to the cannabinoid substance group. Its chemical structure is similar to that of Δ9-tetrahydrocannabinol (Δ9-THC), the main psychoactive cannabinoid in the plant Cannabis sativa L. Until recently, HHC was offered as a legal alternative to cannabis, or Δ9-THC. However, it has since been prohibited in Germany.
- Intake of products containing HHC with the amounts of HHC typically offered for sale are linked to a high probability of the occurrence of health impairments in the form of psychoactive effects.
- Scientific data on the toxicity of HHC is still incomplete. According to current insights, the intake of larger amounts might lead to the occurrence of severe poisoning.
Risk profile for products containing HHC
- How does HHC enter the body?
- Is there a health-based guidance value?
- Is there a health risk?
- How high is the data quality?
- How can the health risk posed by HHC be decreased?
1 Subject of the assessment
Products containing hexahydrocannabinol (HHC) that can be perceived as foodstuffs by consumers (e.g. in the form of wine-gum-like products) are currently illegally available on the German market. The German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment) has therefore carried out a toxicological assessment of HHC in foodstuffs.
2 Results
Hexahydrocannabinol (HHC) has been found in various products available on the European market since 2022. However, since 21/06/2024, HHC has been subject to Germany’s New Psychoactive Substances Act (NpSG). Accordingly, HHC may no longer be manufactured, placed on the market, sold, or purchased in Germany. Possession is not permitted, either. These provisions also apply to products containing HHC. Despite this, illegal products that consumers may perceive as foodstuffs (e.g. wine-gum-like products) remain available. The HHC used in this process is probably produced semi-synthetically from cannabidiol (CBD).
HHC has yet to be sufficiently toxicologically characterised. There is a particular lack of data in regard to acute or chronic toxicity of the substance. There are also only few robust findings related to the effects of HHC on humans. Findings from animal experiments, in vitro studies, case studies in the literature, anecdotal reports of HHC users on the Internet, and a human study carried out with a small number of healthy participants have led to the following conclusions:
- The available data suggests that β-HHC in particular has psychoactive potential. On the other hand, the cannabimimetic activity of α-HHC seems to be considerably lower.
- There is evidence that the effects of β-HHC are similar to those of Δ9-tetrahydrocannabinol (Δ9-THC), although the potency is probably somewhat lower. This means that somewhat higher doses are required in order to attain an effect comparable to that achieved following intake of Δ9-THC.
- According to the current state of knowledge, the HHC-levels in products that can be perceived as foodstuffs by consumers (e.g. wine-gum-like products with 25 mgshort formilligram/piece) may be sufficient to induce a state of euphoria in those who consume them.
- Due to the differences in the cannabimimetic activity of β-HHC and α-HHC, it is to be expected that the effects after consuming products containing HHC with different epimer contents may differ.
- The current state of knowledge indicates that intake of larger amounts, including accidental intake by children, can lead to severe poisoning.
Products containing HHC can, in principle, also be contaminated with residues from the extraction, synthesis by-products, and other phytocannabinoids as well as residues of the catalysts used in the synthesis. However, whether this results in health risks can be assessed only in individual cases.
3 Rationale
3.1 Background
Hexahydrocannabinol (HHC) appeared on the US drug market in late 2021. In Europe, it was first observed in May 2022; by December 2022, HHC products were found in 70% of EU member states. Initial detection in Germany was in December 2022 based on a customs seizure from June 2022 (Kühnl et al.short foret alii (lat. "and others") 2023). Among other things, HHC was used in liquids for e-cigarettes or offered in the form of HHC oils. However, it is also found in products that consumers may perceive as foodstuffs – including wine-gum-like products and food supplements. HHC was openly offered as a legal substitute for cannabis, or Δ9-tetrahydrocannabinol (Δ9-THC) (EMCDDA 2023).
In the EU, HHC is under observation by the European Union Drugs Agency (EUDA), formerly the EMCDDA, as a new psychoactive substance. In the spring of 2023, the agency published a comprehensive report on HHC (EMCDDA 2023). In Germany, HHC has been subject to Germany’s New Psychoactive Substances Act (NpSG) since 21/06/2024. This makes it illegal to trade it, to place it on the market, to manufacture it, to transport it into, out of or through the legal jurisdiction of this law, to purchase it, to possess it or to provide it to another person. These provisions also apply to products containing HHC. HHC has since become regulated in other European countries, too, including Denmark, Finland, France, Greece, Austria, Sweden, Switzerland, the Czech Republic, and the United Kingdom.
3.2 Agent
HHC (IUPAC: 6,6,9-trimethyl-3-pentyl-6a,7,8,9,10,10a-hexahydrobenzo[c]chromen-1-ol, CAS: 6692-85-9, molar mass: 316.48 g/mol) was first described in the scientific literature in 1940. The structure resembles that of Δ9-THC, the most psychoactive cannabinoid in Cannabis sativa L. The only distinction is the missing double bond between C9 and C10. HHC can be present stereochemically in the form of the two epimers 9β-HHC and 9α-HHC (Ujváry 2023).
Figure 1: Structural formulas of Δ9-THC, 9β-HHC and 9α-HHC, numbering according to IUPAC
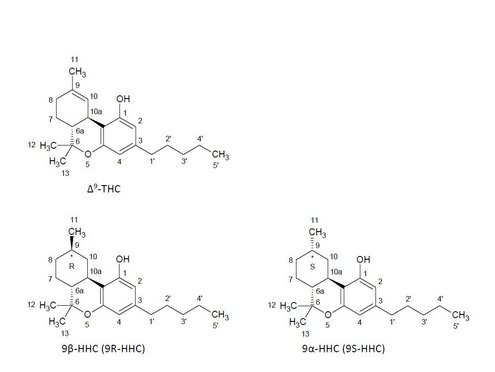
HHC is not naturally biosynthesised in Cannabis sativa L. However, trace amounts of the compound have been detected in the hemp plant. This occurrence is likely a degradation product of Δ9-THC. On a larger scale, production is probably semi-synthetic, starting from cannabidiol (CBD), which is obtained from commercial hemp, among others. The first step in the process is acid-catalysed conversion of CBD into Δ8-THC and Δ9-THC. This is then hydrogenated in a second step. Semi-synthetic production typically results in a mixture of the epimers 9β-HHC and 9α-HHC. However, the ratio can vary depending on the synthesis method. The compound can also be made fully synthetically, although this is probably not particularly common on a larger scale because of the higher costs. Depending on the method of synthesis, different by-products may be formed. The exact production method behind the HHC available on the market is not known (Ujváry 2023).
3.3 Toxicological assessment
HHC has yet to be sufficiently toxicologically characterised. There is a particular lack of data in regard to acute or chronic toxicity of the compound. There are also only few robust findings related to the effects of HHC on humans. Findings from animal experiments, in vitro studies, case studies in the literature, anecdotal reports of HHC users on the Internet, and a human study with a small number of participants indicate that β-HHC in particular mediates effects similar to those of the structurally similar Δ9-THC.
3.3.1 Toxicokinetics
Findings on the toxicokinetics of HHC are limited. Because of the chemical structure, HHC as well as structurally similar cannabinoids can be assumed to be highly lipophilic. This suggests a high absorption rate after oral ingestion, a strong plasma protein binding, and an accumulation in fatty tissue (Ujváry 2023).
Findings from studies on the metabolism of 9β-HHC using microsomal preparations from rat, guinea pig, rabbit, hamster, and mouse showed a hydroxylation pattern similar to that known for Δ9-THC with hydroxylations at C11, C8, and C4 as well as the pentyl side chain (Harvey & Brown 1991). The study focused on monohydroxylated metabolites; other Phase I and Phase II metabolites were not reported.
In a later study, Lindbom et al.short foret alii (lat. "and others") studied the metabolism of HHC following incubation with primary human hepatocytes. In particular, they observed monohydroxylations as well as the following formation of carboxylic compounds. Most metabolites were present in glucuronidated form (Lindbom et al.short foret alii (lat. "and others") 2024). Di Trana et al.short foret alii (lat. "and others") investigated the toxicokinetics of inhalative exposureExposureTo glossary to ~25mg of HHC on six participants. In their saliva, only HHC itself could be detected. In the blood and urine also the metabolites 11-nor-9-carboxy-HHC, 8-hydroxy-HHC, and 11-hydroxy-HHC were found next to the parent substance, which were bound as glucuronic acid conjugates (Di Trana et al.short foret alii (lat. "and others") 2024).
Kobidze et al.short foret alii (lat. "and others") also investigated various matrices for the occurrence of HHC and its metabolites following inhalative exposure to ~25mg of HHC on two participants. They found HHC in the saliva, HHC, 11-nor-9-carboxy-HHC and 11-hydroxy-HHC in the blood and urine, and 9-hydroxy-HHC in the urine. In contrast to the work conducted by Di Trana et al.short foret alii (lat. "and others"), however, they found no 8-hydroxy-metabolites in any matrix (Kobidze et al.short foret alii (lat. "and others") 2024).
Höfert et al.short foret alii (lat. "and others") investigated the toxicokinetics of HHC following oral intake of a wine-gum-like product with 25mg of HHC or following inhalative intake (3 puffs of an inhalation solution with 1mg/mlshort formillilitre of HHC) on three healthy adult participants. Maximum serum concentrations were reached 1.25 to 2 hours after oral intake and 2 to 6 minutes after inhalative exposure. In addition to 9β-HHC and 9α-HHC, the serum also contained the metabolites 11-hydroxy-HHC and 9β-carboxy-HHC. In addition to 9β-HHC (not detectable in all participants) and 9α-HHC, the urine contained the metabolites 11-hydroxy-HHC as well as 9β-carboxy-HHC and 9α-carboxy-HHC. In the saliva, only HHC (both epimers) could be detected, but no metabolites (Höfert et al.short foret alii (lat. "and others") 2025).
3.3.2 Toxicology data
3.3.2.1 Findings from in vitro studies
HHC has been studied in a number of in vitro test systems. In addition to cannabimimetic properties, other endpoints were addressed (e.g. antiproliferative properties in tumour cell lines and binding affinity to opioid receptors). An overview of published studies can be found in the EMCDDA report and in a review paper by Ujváry (EMCDDA 2023; Ujváry 2023). Within the scope of the present opinion, only the essential findings regarding the cannabimimetic (i.e. the Δ9-THC-like) activity of HHC as well as a study on safety pharmacology are described.
A recently published in silico analysis on molecular docking shows that HHC and Δ9-THC should have similar binding affinities to the CB1 and CB2 receptor (Aviz-Amador et al.short foret alii (lat. "and others") 2021) .
Andersson et al.short foret alii (lat. "and others") used HEK 293 cells expressing the human cannabinoid receptors CB1R or CB2R to investigate whether and to what extent 9β-HHC and 9α-HHC (test concentration: 100 μM) lead to an activation of these receptors. Receptor activation was reported only semi-quantitatively as a decrease in forskolin-induced cAMP accumulation. Both epimers caused an activation of CB1R and CB2R in this test system; the effect size was similar to that of the Δ8-THC also investigated (Andersson et al.short foret alii (lat. "and others") 2011).
Another study was recently conducted at the Swedish National Board of Forensic Medicine and is described in the EMCDDA report. CB1R-expressing transfected cells were used in the study. It was shown that 9β-HHC acts as a partial agonist at CB1R (EMCDDA 2023).
In 2023, Nasrallah and Garg published a study that investigated both the binding affinity (radioligand binding assay) and functional activity (G-protein coupled receptor (GPCR) functional assay) of 9β-HHC and 9α-HHC at CB1R and CB2R. This showed that both the binding affinity and the functional activity at CB1R and CB2R for β-HHC are comparable to that of Δ9-THC. In this study, 9α-HHC showed about tenfold lower binding activity and functional activity (Nasrallah & Garg 2023).
A few other studies on CB1R-expressing transfected cells were able to show that 9β-HHC and 9α-HHC lead to activation of the receptor. 9β-HHC fundamentally shows far higher activity than 9α-HHC (Durydivka et al.short foret alii (lat. "and others") 2024; Janssens et al.short foret alii (lat. "and others") 2024; Persson et al.short foret alii (lat. "and others") 2024).
In principle, the comparison of the three-dimensional structure shows that 9β-HHC and Δ9-THC are very similar, while 9α-HHC is very different in parts (Ujváry 2023). Cannabimimetic activity is therefore particularly plausible for 9β-HHC. This observation further supports the experimental results.
In 2022, Collins et al.short foret alii (lat. "and others") published a study in which different endpoints regarding the toxic potential of HHC were addressed. HHC was in this case tested as a mix of the epimers 9β-HHC and 9α-HHC. The mutagenic potential was investigated in a bacterial reverse mutation test (Ames test). From a regulatory perspective, however, the test report is not sufficiently robust. Additionally, the patch-clamp technique was used to examine whether HHC can lead to the deactivation of the hERG channel. As no activity was found here, it is currently not assumed that HHC has QT-time prolonging potential for the heart. Further tests showed that HHC has a cytotoxic effect on human lung fibroblasts (IC50 = 14.4µM), while for human hepatocytes, no relevant cytotoxic effect was observed up to a concentration of 50µM (Collins et al.short foret alii (lat. "and others") 2022).
3.3.2.2 Findings from animal studies
The focus of the animal studies was to clarify the cannabimimetic activity of HHC.
The first studies on this subject date back to the 1940s and addressed the cannabimimetic effect of HHC in the Gayer test (decrease of the corneal reflex in rabbits) (Russell et al.short foret alii (lat. "and others") 1941) and in the ataxia test in dogs (Adams et al.short foret alii (lat. "and others") 1940; Adams et al.short foret alii (lat. "and others") 1942) after intravenous application of the test substance. In both studies, an activity of the test substance that was slightly weaker than THC (approx. 20–50%) was observed. However, the studies are difficult to interpret because the purity and isomeric ratios of HHC and THC were not characterised. In addition, the Gayer test is no longer considered a suitable test for determining cannabimimetic activity (EMCDDA 2023).
The cannabimimetic effect of HHC was later investigated in a comprehensive study on rhesus monkeys. The behaviour of animals after intravenous administration of 9β-HHC (doses: 0.1, 0.5, and 1 mgshort formilligram/kgshort forkilogram body weight (BW)) or 9α-HHC (doses: 1, 2, 5 mgshort formilligram/kgshort forkilogram BW) was assessed using Norton’s score. Administration of 9β-HHC resulted in stupor, ataxia, immobility, and crouched posture as well as reduced response to external stimuli in the animals. The potency of 9β-HHC was about half that of Δ9-THC; the activity of 9α-HHC was about 10-fold lower compared to 9β-HHC. The authors noted that the substances were not completely isotopically pure. The effects of 9α-HHC could thus also have been caused by low levels of 9β-HHC (Edery et al.short foret alii (lat. "and others") 1971; Mechoulam et al.short foret alii (lat. "and others") 1980).
Skinnert et al.short foret alii (lat. "and others") investigated the effects of several cannabinoids on the endpoints locomotor activity, postural arrest, body temperature, and pain sensation (hot plate test) in mice after intraperitoneal application of HHC as a mixture (about 1:1) of the two epimers 9β-HHC and 9α-HHC. The potency of HHC was about one order of magnitude or more lower than that of Δ9-THC depending on the endpoint. However, unlike Δ9-THC, HHC showed no analgesic effect in this study (Skinner et al.short foret alii (lat. "and others") 1979).
Intravenous administration of Δ9-THC is known to induce convulsions in the New Zealand White rabbit (Martin et al.short foret alii (lat. "and others") 1977). Consroe et al.short foret alii (lat. "and others") therefore investigated different cannabinoids in this animal model. Compared with Δ9-THC, HHC showed a potency of about 50% (Consroe et al.short foret alii (lat. "and others") 1982).
In a study published in 2023, Russo et al.short foret alii (lat. "and others") investigated the cannabimimetic potential of 9β-HHC and 9α-HHC after intraperitoneal application to mice in the tetrad test (locomotor activity, catalepsy, body temperature, pain sensation; dose: 10 mgshort formilligram/kgshort forkilogram BW). A non-significant cataleptic effect as well as a non-significant decrease in body temperature was observed in the 9β-HHC group. In addition, a significant analgesic effect and a significant decrease in locomotor activity were observed in this group. In contrast, there was no relevant change in the animals treated with 9α-HHC compared with the control animals. In this study, no Δ9-THC group was included (Russo et al.short foret alii (lat. "and others") 2023).
Recently, Marusich et al.short foret alii (lat. "and others") also investigated the cannabimimetic potential of 9β-HHC and 9α-HHC following intraperitoneal application on mice with the tetrad test (doses: 10, 30, 100 mgshort formilligram/kgshort forkilogram KG) and the drug discrimination test (doses: 0,3, 1, 3, 10 mgshort formilligram/kgshort forkilogram KG for 9β-HHC; 10, 30, 56, 100 mgshort formilligram/kgshort forkilogram KG for 9α-HHC) compared to Δ9-THC. In the tetrad test, 9β-HHC showed similar cannabimimetic activity to Δ9-THC, while 9α-HHC was far less potent and only showed slight activity for two of the four parameters. The cannabimimetic stimulus in the drug discrimination test was also comparable for 9β-HHC and Δ9-THC, while 9α-HHC was far less potent here, too. After applying the higher doses of 30 and 10 mgshort formilligram/kgshort forkilogram KG of 9β-HHC, further acutely toxic effects were also shown in some mice. These included seizures, shaking, and muscle tension. After 5 or 6 days, respectively, four of the eight mice from the tetrad test group who had received 100 mgshort formilligram/kgshort forkilogram KG 9β-HHC were found dead. In the view of the authors, it is unclear if this was due to a treatment-related effect (Marusich et al.short foret alii (lat. "and others") 2025).
Currently, there is still a lack of studies on classic toxicological endpoints related to acute and chronic toxicity of HHC.
3.3.2.3 Findings from studies on participants
Höfert et al.short foret alii (lat. "and others") investigated the psychoactive effects of HHC following oral intake of a wine-gum-like product with 25 mgshort formilligram of HHC or following inhalative intake (3 puffs of an inhalation solution with 1 mgshort formilligram/mlshort formillilitre of HHC) on three healthy adult participants. Here, all participants from the oral intake group and two of the three participants from the inhalation group reported a subjectively perceived feeling of euphoria. The extent of the euphoria varied greatly between participants, particularly following inhalative exposure. Further tests (modified Romberg test, nystagmus test, pupil size, walk-and-turn test, one-leg stand test, finger-finger test, finger-nose test) only partially showed indications of HHC-related changes. All participants reported dry mouth. It should be noted that the study only included three participants per application type and was neither blinded nor placebo-controlled (Höfert et al.short foret alii (lat. "and others") 2025).
3.3.2.4 Insights from experiential reports from consumers and expert surveys
Between June and August 2023, as part of the German National Early Warning System (NEWS), information on HHC was collected and consolidated and then published in the Trendspotter Report (September 2023) (Kühnl et al.short foret alii (lat. "and others") 2023). Data collection included surveying experts and consumers on the topic of HHC. Generally, the Trendspotter Report describes a somewhat weaker psychoactive effect of HHC compared to THC. At the same time, however, consumers of HHC report an array of in some cases severe, undesired physical and psychological effects. Due to the frequently reported mixed consumption, the effects and side-effects following consumption of HHC are difficult to determine. Furthermore, experiential reports are based almost exclusively on self-reported HHC consumption which cannot be independently verified. As such, there is no evidence as to whether persons actually consumed HHC or in fact consumed products falsely labelled as HHC which contained other substances, such as THC or synthetic cannabinoids. The results can therefore not be used to draw scientifically robust conclusions.
3.3.2.5 Case studies
Case studies from France, the Czech Republic, and Germany indicate that intake of HHC or products likely containing HHC can lead to light to severe symptoms (Holt 2024).
A retrospective observational study describes self-reported HHC exposure (n = 37) which led to calls to French poison information centres between January 2022 and May 2023 (Labadie et al.short foret alii (lat. "and others") 2024). The severity was classified as light in 40% of cases, moderate in 43%, and severe in 5% (2 cases). The majority of patients showed neurological and cardiovascular symptoms.
In five of six tested cases, HHC was detected in blood and/or urine using liquid chromatography–tandem-mass spectrometry (LC-MS/MS). In three cases, only HHC was detected, while in two cases THC and metabolites were also detected. In the sixth case, no HHC could be detected in the blood (sample taken after >24 h), although HHC, THC, and CBD were found in the product.
For the three cases in which only HHC was detected, a variety of symptoms were reported (neurological, gastrointestinal, cardiovascular, ocular, and psychiatric symptoms). The severity was classified as moderate in two cases and as severe in one case.
Information on cases (n = 236, of which 38 with detected cannabinoids) reported to the Czech poison information centres between May 2022 and April 2024 also show that various symptoms can occur following exposure to HHC.
In Germany, the Trendspotter Report published data from three poison information centres. They had been contacted 25 times regarding HHC exposure in the time period from late 2022 to July 2023. In the nine cases for which information regarding the degree of severity was reported, five cases were classified as moderate.
The Trendspotter Report includes a case toxicologically verified by experts in which children lost consciousness following accidental consumption of wine gums containing HHC which were also labelled accordingly (Kühnl et al.short foret alii (lat. "and others") 2023).
By now, there have also been case reports about the occurrence of psychoses linked to the consumption of HHC (Oshort foroxygen'Mahony et al.short foret alii (lat. "and others") 2024).
In general, however, it should be noted that only a few of the reported cases have been analytically verified. Overall, possible health effects of HHC cannot currently be conclusively gauged.
3.3.2.6 Other toxicological aspects
The exact synthesis methods and manufacturing conditions of HHC are not known for individual products. However, it can generally be assumed that HHC is primarily obtained semi-synthetically from CBD. The conversion of CBD to Δ8-THC and Δ9-THC is the first step. Numerous studies have shown that various by-products are also formed. The exact pattern of the resulting products differs depending on the exact manufacturing conditions. Unless adequate purification takes place, the final HHC products may be contaminated with residues from the extraction, by-products, and other phytocannabinoids as well as residues from the catalysts used. However, there is no analytical data available for such products so far (EMCDDA 2023; Ujváry 2023). Whether this results in health risks can be assessed only in individual cases.
Furthermore, it must be taken into account that the purity of HHC products may deviate from the manufacturer’s specifications. For example, one HHC product sold in the US contained Δ8-THC, Δ9-THC, Δ6a,10a-THC, but no HHC (Sams 2020).
3.3.3 Exposure
The BfRshort forGerman Federal Institute for Risk Assessment does not yet have comprehensive knowledge about the levels of HHC in products that might be perceived as foodstuffs by consumers. Two products presented in the EMCDDA report have a content of 25 mgshort formilligram per wine gum or marshmallow according to the product declaration (EMCDDA 2023). A cursory Internet search yielded numerous hits for other products with the HHC content often stated as 25 mgshort formilligram per wine gum or higher.
3.3.4 Miscellaneous
Due to the structural similarity of the compounds, the use of immunological rapid tests for detecting Δ9-THC and its metabolites can also lead to positive results in the presence of HHC and its metabolites. There appears to be pronounced cross reactivity here. Sensitivity can vary depending on the substance and the test (Wolf et al.short foret alii (lat. "and others") 2023; Derne et al.short foret alii (lat. "and others") 2024; Helander et al.short foret alii (lat. "and others") 2024; Höfert et al.short foret alii (lat. "and others") 2024; Kronstrand et al.short foret alii (lat. "and others") 2024; Patton et al.short foret alii (lat. "and others") 2024).
3.4 Risk management options, recommended measures
HHC has yet to be sufficiently toxicologically characterised. There is a particular lack of data in regard to acute or chronic toxicity of the substance. There are also only few findings related to the effects of HHC on humans. Findings from animal experiments, in vitro studies, anecdotal reports of HHC users on the Internet, and a human study carried out with a small number of healthy participants have led to the following conclusions:
- The available data suggests that β-HHC in particular has psychoactive potential. On the other hand, the cannabimimetic activity of α-HHC, seems to be considerably lower.
- There is evidence that the effects of β-HHC are similar to those of Δ9-tetrahydrocannabinol (Δ9-THC), although the potency is probably somewhat lower. This means that somewhat higher doses are required in order to attain an effect comparable to that achieved following intake of Δ9-THC.
- According to the current state of knowledge, the HHC-levels in products that can be perceived as foodstuffs by consumers (e.g. wine-gum-like products with 25 mgshort formilligram/piece) may be sufficient to induce a state of euphoria in the consumer.
- Due of the differences in the cannabimimetic activity of β-HHC and α-HHC, it is to be expected that the effects after consumption of products containing HHC with different epimer contents may differ.
- The current state of knowledge indicates that intake of larger amounts, including accidental intake by children, can lead to severe poisoning.
- Products containing HHC can, in principle, also be contaminated with residues from the extraction, synthesis by-products, and other phytocannabinoids as well as residues of the catalysts used in the synthesis. However, whether this results in health risks can be assessed only in individual cases.
- It should be noted that HHC has been subject to Germany’s New Psychoactive Substances Act (NpSG) since 21/06/2024. HHC may therefore not be manufactured, placed on the market, sold, or purchased in Germany. Possession is not permitted, either. These provisions also apply to products containing HHC.
Further information on the BfRshort forGerman Federal Institute for Risk Assessment website on substance risks in foods
- Topic page on the assessment of substance risks in foods To the page
- Questions and answers on the health risks of food and feed containing hemp To the FAQ
4 References
Adams R., Loewe S., Pease D. C., Cain C. K., Wearn R. B., Baker B. R., Wolff H. (1940). Structure of cannabidiol. VIII. Position of the double bonds in cannabidiol. Marihuana activity of tetrahydrocannabinols. Journal of the American Chemical Society 62: 2566.
Adams R., Loewe S., Smith C. M., McPhee W. D. (1942). Tetrahydrocannabinol Homologs and Analogs with Marihuana Activity. XIII. Journal of the American Chemical Society 64: 694-697.
Andersson D. A., Gentry C., Alenmyr L., Killander D., Lewis S. E., Andersson A., Bucher B., Galzi J. L., Sterner Oshort foroxygen., Bevan S., Hogestatt E. D., Zygmunt Pshort forphosphorus. M. (2011). TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ9-tetrahydrocannabiorcol. Nature Communications 2: 551.
Aviz-Amador A., Contreras-Puentes N., Mercado-Camargo J. (2021). Virtual screening using docking and molecular dynamics of cannabinoid analogs against CB(1) and CB(2) receptors. Computational Biology and Chemistry 95: 107590.
Collins A. C., Tesfatsion T. T., Ramirez G. A., Ray K. Pshort forphosphorus., Cruces W. (2022). Nonclinical In Vitro Safety Assesment Summary of Hemp Derived (R/S)-Hexahydrocannabinol ((R/S)-HHC). Research Square.
Consroe Pshort forphosphorus., Martin A. R., Schneiderman Fish B. (1982). Use of a potential rabbit model for structure-behavioral activity studies of cannabinoids. Journal of Medicinal Chemistry 25: 596-599.
Derne A. S., Pape E., Jouzeau J. Y., Kolodziej A., Gambier N., Scala-Bertola J. (2024). Immunological detection of hexahydrocannabinol (HHC) in oral fluid. Drug Testing and Analysis 16: 638-640.
Di Trana A., Di Giorgi A., Sprega G., Carlier J., Kobidze G., Montanari E., Taoussi Oshort foroxygen., Bambagiotti G., Fede M. S., Lo Faro A. F., Tini A., Busardò F. Pshort forphosphorus., Pichini S. (2024). Disposition of Hexahydrocannabinol Epimers and Their Metabolites in Biological Matrices following a Single Administration of Smoked Hexahydrocannabinol: A Preliminary Study. Pharmaceuticals 17.
Durydivka Oshort foroxygen., Palivec Pshort forphosphorus., Gazdarica M., Mackie K., Blahos J., Kuchar M. (2024). Hexahydrocannabinol (HHC) and Δ9-tetrahydrocannabinol (Δ9-THC) driven activation of cannabinoid receptor 1 results in biased intracellular signaling. Scientific Reports 14.
Edery H., Grunfeld Y., Ben-Zvi Z., Mechoulam R. (1971). Structural requirements for cannabinoid activity. Annals of the New York Academy of Sciences 191: 40-53.
EMCDDA (European Monitoring Centre for Drugs and Drug Addiction) (2023). Hexahydrocannabinol (HHC) and related substances. Technical Report: 1-108. External Link:https://www.emcdda.europa.eu/system/files/documents/2023-05/emcdda-technical-report-hhc-and-related-substances.pdf.
Harvey D. J. and Brown N. K. (1991). Comparative in vitro metabolism of the cannabinoids. Pharmacology, Biochemistry and Behavior 40: 533-540.
Helander A., Johansson M., Villén T., Andersson A. (2024). Appearance of hexahydrocannabinols as recreational drugs and implications for cannabis drug testing–focus on HHC, HHC-Pshort forphosphorus, HHC-Oshort foroxygen and HHC-H. Scandinavian Journal of Clinical and Laboratory Investigation 84: 125-132.
Höfert L., Becker S., Dreßler J., Baumann S. (2024). Quantification of (9R)- and (9S)-hexahydrocannabinol (HHC) via GC–MS in serum/plasma samples from drivers suspected of cannabis consumption and immunological detection of HHC and related substances in serum, urine, and saliva. Drug Testing and Analysis 16: 489-497.
Holt E. (2024). Czech Republic latest country to ban hexahydrocannabinol. The Lancet 403: 604.
Janssens L. K., Van Uytfanghe K., Williams J. B., Hering K. W., Iula D. M., Stove C. Pshort forphosphorus. (2024). Investigation of the intrinsic cannabinoid activity of hemp-derived and semisynthetic cannabinoids with β-arrestin2 recruitment assays—and how this matters for the harm potential of seized drugs. Archives of Toxicology 98: 2619-2630.
Kobidze G., Sprega G., Montanari E., Taoussi Oshort foroxygen., Bambagiotti G., Fede M. S., Di Trana A., Pichini S., Busardò F. Pshort forphosphorus., Tini A., Chankvetadze B., Faro A. F. L. (2024). The first LC-MS/MS stereoselective bioanalytical methods to quantitatively detect 9R- and 9S-hexahydrocannabinols and their metabolites in human blood, oral fluid and urine. Journal of Pharmaceutical and Biomedical Analysis 240.
Kronstrand R., Roman M., Green H., Truver M. T. (2024). Quantitation of hexahydrocannabinol (HHC) and metabolites in blood from DUID cases. J Anal Toxicol 48: 235-241.
Kühnl R., Bergmann H., Mathäus F., Janz M., Neumeier E., IFT (Institut für Therapieforschung) (2023). Hexahydrocannabinol (HHC) - Trendspotter. September: 1-27. External Link:https://mindzone.info/wp-content/uploads/2023/09/NEWS-Trendspotter_HHC.pdf.
Labadie M., Nardon A., Castaing N., Bragança C., Daveluy A., Gaulier J. M., El Balkhi S., Grenouillet M. (2024). Hexahydrocannabinol poisoning reported to French poison centres. Clinical Toxicology 62: 112-119.
Lindbom K., Norman C., Baginski S., Krebs L., Stalberga D., Rautio T., Wu X., Kronstrand R., Gréen H. (2024). Human metabolism of the semi-synthetic cannabinoids hexahydrocannabinol, hexahydrocannabiphorol and their acetates using hepatocytes and urine samples. Drug Testing and Analysis.
Martin B. R., Dewey W. L., Aceto M. D., Adams M. D., Earnhardt J. T., Carney J. M. (1977). A potent antinociceptive cannabinoid which lacks opiate substitution properties in monkeys. Research Communications in Chemical Pathology and Pharmacology 16: 187-190.
Mechoulam R., Lander N., Varkony T. H., Kimmel I., Becker Oshort foroxygen., Ben-Zvi Z., Edery H., Porath G. (1980). Stereochemical Requirements for Cannabinoid Activity. Journal of Medicinal Chemistry 23: 1068-1072.
Nasrallah D. J. and Garg N. K. (2023). Studies Pertaining to the Emerging Cannabinoid Hexahydrocannabinol (HHC). ACS Chemical Biology.
Oshort foroxygen'Mahony B., Oshort foroxygen'Malley A., Kerrigan Oshort foroxygen., McDonald C. (2024). HHC-induced psychosis: A case series of psychotic illness triggered by a widely available semisynthetic cannabinoid. Irish Journal of Psychological Medicine: 1-4.
Patton A. L., Pacheco I. C., Seither J. Z., Brown J. T., Walterscheid J. Pshort forphosphorus., Karschner E. L. (2024). Cross-reactivity of 24 cannabinoids and metabolites in blood using the Immunalysis Cannabinoids Direct enzyme-linked immunosorbent assay. J Anal Toxicol 48: 439-446.
Persson M., Kronstrand R., Evans-Brown M., Green H. (2024). In vitro activation of the CB1 receptor by the semi-synthetic cannabinoids hexahydrocannabinol (HHC), hexahydrocannabinol acetate (HHC-Oshort foroxygen) and hexahydrocannabiphorol (HHC-Pshort forphosphorus). Drug Testing and Analysis.
Russell Pshort forphosphorus. B., Todd A. R., Wilkinson S., Macdonald A. D., Woolfe G. (1941). Cannabis indica. VII. The relation between chemical constitution and hashish activity. Journal of the Chemical Society (Resumed): 169-172.
Russo F., Vandelli M. A., Biagini G., Schmid M., Luongo L., Perrone M., Ricciardi F., Maione S., Laganà A., Capriotti A. L., Gallo A., Carbone L., Perrone E., Gigli G., Cannazza G., Citti C. (2023). Synthesis and pharmacological activity of the epimers of hexahydrocannabinol (HHC). Scientific reports 13: 11061.
Sams R. A. (2020). Analysis of Hexahydrocannabinols: Eliminating UncertaintyUncertaintyTo glossary in its Identification. External Link:https://forgehemp.com/wp-content/uploads/2022/03/Analysis-of-Hexahydrocannabinols-280222.pdf.
Skinner W. A., Rackur G., Uyeno E. (1979). Structure‐activity studies on tetrahydro‐ and hexahydrocannabinol derivatives. Journal of Pharmaceutical Sciences 68: 330-332.
Ujváry I. (2023a). Hexahydrocannabinol and closely related semi-synthetic cannabinoids: A comprehensive review. Drug Testing and Analysis.
Ujváry I. (2023b). Hexahydrocannabinol and closely related semi-synthetic cannabinoids: A comprehensive review. Drug Testing and Analysis: 1-35.
Wolf C. E., Pokhai A. A., Poklis J. L., Williams G. R. (2023). The cross-reactivity of cannabinoid analogs (delta-8-THC, delta-10-THC and CBD), their metabolites and chiral carboxy HHC metabolites in urine of six commercially available homogeneous immunoassays. J Anal Toxicol 47: 732-736.
About the BfRshort forGerman Federal Institute for Risk Assessment
The German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment) is a scientifically independent institution within the portfolio of the Federal Ministry of Food and Agriculture (BMELshort forGerman Federal Ministry of Food and Agriculture) in Germany. The BfRshort forGerman Federal Institute for Risk Assessment advises the Federal Government and the States (‘Laender’) on questions of food, chemicals and product safety. The BfRshort forGerman Federal Institute for Risk Assessment conducts independent research on topics that are closely linked to its assessment tasks.
This text version is a translation of the original German text, which is the only legally binding version.