Metal kitchen utensils: Do substances transfer into food? Results from regional authorities give no cause for concern
What it's about:
- In many kitchens, uncoated and enamelled metal items such as pots, pans and cutlery come into daily contact with food. Small amounts of elements from the materials can transfer into food and thus be ingested by humans.
- There are currently no legal limit values in the EU for the release of elements from uncoated and enamelled metal items into food. However, there is a technical guide from the Council of Europe on metals and alloys and a technical standard for enamelled items in contact with food.
- In 2022, 194 metal items, both uncoated and enamelled, were examined as part of a nationwide monitoring programme. The regional authorities of the German federal states ("Laender") investigated which elements can be transferred from the materials into food in which amounts.
- The German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment) evaluated the results of this study and assessed whether the amounts released could pose health risks. To this end, the daily intake amounts were estimated based on the element release measurements. These were then compared with health-based guidance values (HBGVshort forHealth-Based Guidance Value) or toxicological reference values.
- Most of the items examined released only very small amounts of the elements in question. It is the opinion of the BfRshort forGerman Federal Institute for Risk Assessment that these products are therefore suitable for contact with food.
- However, some items contributed substantially to people’s daily intake of certain elements, especially when other sources such as food were also taken into account. The BfRshort forGerman Federal Institute for Risk Assessment recommends that manufacturers of such items improve materials and production processes in order to further reduce the release of elements into food.
- Only a few items released elements to a degree that could exceed the derived HBGVshort forHealth-Based Guidance Value or toxicological reference values and thus increase the risk of the occurrence of health impairments. From a toxicological point of view, these items are not suitable for contact with food.
Risk profile
- How do substances from metal kitchen utensils enter the body?
- Is there a health-based guidance value?
- Is there a health risk?
- How can the health risk be reduced?
- How can the health risk be reduced?
1 Introduction
Uncoated and enamelled metal items intended to come into contact with food are ubiquitous in the kitchen and include pots, pans, baking trays, and cutlery. If elements are released from these objects, they can migrate into food and be ingested. Currently, there are no harmonised legal limit values in the EU for the release of elements from these metal items. However, there are recommendations from the European Directorate for the Quality of Medicines for the release from uncoated metal food contact materials (EDQM, 2024) and the standard DIN EN ISO 4531:2022 for enamelled items for contact with food.
In 2022, the German regional authorities of the German federal states ("Laender") investigated the release of elements from metal food contact materials as part of the monitoring programme. The release of 21 different elements from a total of 194 uncoated or enamelled metal objects was investigated.
The German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment) used the results of the monitoring programme as an opportunity for health risk assessment of the potential intake of the released elements through food. To this end, the estimated daily intake of the elements from the released amounts was compared with the health-based guidance values (HBGVshort forHealth-Based Guidance Value) or toxicological reference values derived from current studies.
A comparison of the release quantities with the maximum values from the above-mentioned standard and the technical guide provided by the Council of Europe showed that the vast majority of uncoated and enamelled metal items release only small amounts of elements. The risk assessment based on the exposureExposureTo glossary assessment carried out showed that these items are suitable for food contact. However, with regard to overall exposure, taking into account possible additional sources of intake (such as food), the BfRshort forGerman Federal Institute for Risk Assessment considers that some objects contribute too much to the daily intake of certain elements. The BfRshort forGerman Federal Institute for Risk Assessment recommends that manufacturers of these products review their raw materials and manufacturing processes in order to further reduce element release. Only a few items showed element releases that could exceed the derived HBGVshort forHealth-Based Guidance Value or toxicological reference values and thus increase the risk of the occurrence of health impairments. From a toxicological point of view, these items are not suitable for contact with food.
2 Subject of the assessment
In 2022, regional authorities from ten German federal states ("Laender") investigated uncoated and enamelled metal kitchen items with regard to the possible release of elements into food. Currently, there are no legal limit values in the EU for the release of elements from metal food contact materials or enamelled food contact materials. However, according to legal requirements, food contact materials must comply with current technical standards and must not release substances into food in amounts that could pose a health risk to consumers. In its recently updated technical guide on metals and alloys in food contact, the Council of Europe has derived specific release limits (SRLs) for a large number of elements as an interpretation of this general legal provision (EDQM, 2024). For enamelled metal items, the recently revised standard DIN EN ISO 4531:2022 applies, which contains release limits for enamelled items in contact with food (DIN EN ISO, 2022).
The German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment) has taken the monitoring results as an opportunity to assess the release of the 21 analysed elements from metallic food contact materials in accordance with the latest toxicological findings.
3 Result
To assess the proper manufacture of uncoated metal items, the element releases in the third consecutive migration test were compared with the SRLs of the Council of Europe technical guide on metals and alloys in food contact published in 2024 (EDQM, 2024). 99.1% of the 115 samples tested demonstrated compliance with these assessment values in the third migration test. Only one sample (cast iron pan) was conspicuous and showed increased releases of iron and cobalt. Element releases in the third migrant are used for the assessment of multi-use items (such as cutlery, pans, cups, etc.), as it more realistically reflects the long-term, repeated release of substances than the first migrant.
The limit values for element release specified in DIN EN ISO 4531:2022 were used to assess the proper manufacture of enamelled products. Here, the release quantities from 53.2% of the 79 samples tested in the third migrant were below all respective limit values.
It should be noted that, out of a total of 194 samples, 24 samples exceeded the respective limit value for the release of a single element and 14 samples exceeded the respective limit values for the release of several elements. Aluminium release exceeded the limit value most frequently (33 samples). The high overall number of samples (80.4%) that demonstrate compliance with all release limits clearly shows that it is possible to manufacture such products with low element releases. Manufacturers of products with excessive element releases should review and adjust their raw materials and production processes accordingly in order to further reduce element releases.
An exposure assessment was carried out based on the element releases from the respective metal objects in the third migration. For a risk assessment, exposure to the released elements was then compared with the respective tolerable daily intake (TDIshort forTolerable Daily Intake) or comparable health-based guidance values (HBGVshort forHealth-Based Guidance Value). If the intake of the elements from sources other than food contact materials already almost or completely exhausts the respective HBGVshort forHealth-Based Guidance Value, additional allocation factors were included in the consideration. No HBGVshort forHealth-Based Guidance Value could be derived for arsenic, beryllium, lead and thallium. For beryllium and thallium, this was due to insufficient data and for arsenic and lead the reason was that, according to the current state of research, no intake level without adverse health effects is known for these elements. In the EU, the ALARA principle ("As Low As Reasonably Achievable") is to be applied for risk management in such cases. In the event of an unavoidable release of these elements from food contact materials, resulting intake levels were identified that should not be exceeded under any circumstances.
In 186 of 194 samples tested, the element releases did not result in any exceedance of an HBGVshort forHealth-Based Guidance Value or toxicological reference value. In the BfR’s opinion, the remaining 8 items should not be used for contact with food. However, when allocation factors were applied, the calculated exposure values for 39 samples (20.1%) exceeded the assigned HBGVshort forHealth-Based Guidance Value for one or more elements or the values considered to be the maximum unavoidable exposure to arsenic or lead. In most of these samples, the occurrence of exceedances occurred for only a single element, while in 11 samples (5.7%), several elements were affected. Since the use of these objects can contribute significantly to the overall intake of some elements, the BfRshort forGerman Federal Institute for Risk Assessment believes that manufacturers should revise their raw material qualities and manufacturing processes so that exposure from these food contact materials is below the allocated HBGVshort forHealth-Based Guidance Value for all elements and below an exposure contribution of 0.003 mgshort formilligram lead/person/day and 0.00036 mgshort formilligram arsenic/person/day.
However, the vast majority of uncoated and enamelled metallic materials showed low to very low element release and are suitable for food contact.
4 Rationale
4.1 Risk assessment
[Translate to Englisch:]
4.1.1 Hazard identification
Metal kitchenware, especially enamelled kitchenware, may contain a variety of different elements due to the manufacturing process, including heavy metals such as lead, cadmium and cobalt. When in contact with food, these elements may be released from the metal objects and transferred to the food. Excessive intake of such elements by humans may result in an increased risk of the occurrence of health impairments. The type and amount of elements released depend on various factors, including the composition of the contact material and the material quality of the items, the temperature and type of use, the type of food (e.g. acidic, liquid or solid food) and the duration of contact between the metal object and the food.
4.1.2 Hazard characterisation
In order to determine the amounts in which elements can be released from such metal objects during use, release tests were carried out for 21 different elements. The results of the release tests show that most of the consumer goods tested release only small amounts of elements or even that the element releases were below the limit of quantification or detection. Nevertheless, in order to provide a comprehensive picture, the toxicological properties of all tested elements are presented below, regardless of the extent of release. In addition, exposure to these elements from other sources, such as food, is taken into account. If intake from such sources already largely or completely exhausts the respective HBGVshort forHealth-Based Guidance Value or toxicological reference value, additional allocation factors are included in the assessment. There are a wide variety of approaches to the use of allocation factors worldwide (Greene et al, 2025). In this opinion, an allocation factor of 10% was used if the respective HBGVshort forHealth-Based Guidance Value was more than 50% exhausted by intake from other sources, and 20% if the exhaustion was more than 10%. If other sources of intake are known but the intake from these sources is less than 10% of the HBGVshort forHealth-Based Guidance Value, no allocation factor was used. For arsenic, lead and thallium, for which no HBGVshort forHealth-Based Guidance Value could be derived for various reasons, an allocation factor of 10% of the respective toxicological reference value was used.
4.1.2.1 Aluminium
According to the current state of research, aluminium is neither genotoxic nor carcinogenic (COT, 2013; EFSAshort forEuropean Food Safety Authority, 2008). However, it is neurotoxic, nephrotoxic and toxic to reproduction (SCCS, 2014). Developmental neurotoxicity is considered the most critical endpoint (EFSAshort forEuropean Food Safety Authority, 2008). Aluminium is poorly absorbed after oral intake, usually less than 1% (BfRshort forGerman Federal Institute for Risk Assessment, 2019). It is distributed throughout all tissues, with accumulation occurring particularly in the bones (COT, 2013; EFSAshort forEuropean Food Safety Authority, 2008; JECFA, 2012). Due to its accumulation behaviour and the adverse effects described, the European Food Safety Authority (EFSAshort forEuropean Food Safety Authority) derived a tolerable weekly intake (TWI) of 1 mgshort formilligram/kgshort forkilogram body weight (bw)/week instead of a tolerable daily intake (TDIshort forTolerable Daily Intake) for aluminium (EFSAshort forEuropean Food Safety Authority, 2008). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) has derived a provisional TWI (PTWI) of 2 mgshort formilligram/kgshort forkilogram bw/week (JECFA, 2012).
The main sources of exposure to aluminium are food and drinking water. EFSAshort forEuropean Food Safety Authority estimates the average weekly aluminium intake for an adult to be 0.2 to 1.5 mgshort formilligram/kgshort forkilogram bw/week (EFSAshort forEuropean Food Safety Authority, 2008). Dermal intake of aluminium from cosmetic products such as antiperspirants contributes little to overall exposure to aluminium (BfRshort forGerman Federal Institute for Risk Assessment, 2023). Since intake of aluminium via food can already exhaust the EFSAshort forEuropean Food Safety Authority TWI of 1 mgshort formilligram/kgshort forkilogram bw/week, the release of aluminium from food contact materials should be kept to a minimum. An allocation factor of 10% is considered appropriate for the contribution from food contact materials, in line with Regulation (EU) No 10/2011. The release of aluminium from food contact materials should be as low as possible, but in the BfR’s opinion, an oral intake of 6 mgshort formilligram/week of aluminium from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram.
4.1.2.2 Antimony
EFSAshort forEuropean Food Safety Authority has assessed antimony as non-genotoxic (EFSAshort forEuropean Food Safety Authority, 2004). However, antimony trioxide is classified as a possible carcinogen (Carc. 2) in accordance with the Regulation on Classification, Labelling and Packaging (CLP Regulation). The World Health Organisation (WHO) derived a TDIshort forTolerable Daily Intake from an oral subchronic animal study with antimony trioxide (Poon et al.short foret alii (lat. "and others"), 1998). The NOAEL ("no observed adverse effect level") of 6 mgshort formilligram/kgshort forkilogram body weight was used to derive the TDIshort forTolerable Daily Intake of 6 µgshort formicrogram/kgshort forkilogram bw/day (WHO, 2003; WHO, 2022; Lynch et al.short foret alii (lat. "and others"), 1999).
The French Agency for Food, Environmental and Occupational Health & Safety (ANSES) estimates the average daily intake of antimony through food to be 0.03 µgshort formicrogram/kgshort forkilogram bw/day for adults and 0.04 µgshort formicrogram/kgshort forkilogram bw/day for children (ANSES, 2011). Accordingly, dietary exposure is well below the TDIshort forTolerable Daily Intake, and the application of an allocation factor for the contribution from food contact materials is not necessary. In the BfRshort forGerman Federal Institute for Risk Assessment's view, an oral intake of 0.36 mgshort formilligram antimony/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram.
4.1.2.3 Arsenic
According to the current state of research, arsenic and inorganic arsenic compounds are carcinogenic and toxic to reproduction (EFSAshort forEuropean Food Safety Authority, 2024 a; IARC, 2012; the BfRshort forGerman Federal Institute for Risk Assessment, 2015). They also induce DNA damage, in particular clastogenic and aneugene effects (EFSAshort forEuropean Food Safety Authority, 2024 a). As there is no trigger threshold for genotoxic-carcinogenic effects, the "margin of exposure" approach is used for risk assessment. This involves calculating the margin between the daily intake and a toxicological reference point. EFSAshort forEuropean Food Safety Authority used the "benchmark dose lower confidence limit" (BMDLshort forBenchmark Dose Lower Confidence Limit) based on an epidemiological study on skin cancer as a reference point (EFSAshort forEuropean Food Safety Authority, 2024 a). This BMDLshort forBenchmark Dose Lower Confidence Limit represents the lower confidence limit of what is known as a benchmark dose (BMDshort forBenchmark Dose), meaning the dose at which a certain change would be observed compared to the control. For the study mentioned, EFSAshort forEuropean Food Safety Authority 2024 derived a BMDLshort forBenchmark Dose Lower Confidence Limit05 of 0.06 μg inorganic arsenic/kgshort forkilogram bw/day (EFSAshort forEuropean Food Safety Authority, 2024 a). Due to its genotoxic and carcinogenic effects, arsenic intake should be as low as possible.
Arsenic is mainly ingested through food and is found in particular in rice and other grains. For rice (products), Regulation (EU) 2023/915 sets maximum levels between 0.03 and 0.30 mgshort formilligram inorganic arsenic/kgshort forkilogram fresh weight. For fruit juices and baby food, the maximum levels are between 0.01 and 0.02 mgshort formilligram/kgshort forkilogram. According to EFSAshort forEuropean Food Safety Authority (2021), the average dietary exposure to arsenic for adults is between 0.03 and 0.15 µgshort formicrogram/kgshort forkilogram bw/day. In its opinion, EFSAshort forEuropean Food Safety Authority did not make any recommendations as to what margin of exposure would be sufficiently safe. In any case, exposure through food already leads to very low margins of exposure. In general, arsenic intake should be as low as possible. The release of arsenic from food contact materials should therefore be as low as technically possible, and food contact materials should not contribute significantly to arsenic exposure in the opinion of the BfRshort forGerman Federal Institute for Risk Assessment. As part of a pragmatic approach in cases of unavoidable arsenic exposure from food contact materials, these should not exceed 10% of the above-mentioned BMDLshort forBenchmark Dose Lower Confidence Limit05 (corresponding to an intake of 0.36 µgshort formicrogram/day for a person weighing 60 kgshort forkilogram).
4.1.2.4 Barium
Barium can cause cardiovascular effects after oral intake, but the most sensitive endpoints are nephropathies (Kravchenko et al.short foret alii (lat. "and others"), 2014; EU-FORA, 2022). The BfRshort forGerman Federal Institute for Risk Assessment considers the TDIshort forTolerable Daily Intake of 0.2 mgshort formilligram barium/kgshort forkilogram bw/day derived by the Agency for Toxic Substances and Disease Registry (ATSDR) and recognised by the Scientific Committee on Health and Environmental Risks (SCHER) to be appropriate for risk assessment (ATSDR, 2007; SCHER, 2012). The derivation was based on a chronic study in mice (reference point: BMDLshort forBenchmark Dose Lower Confidence Limit05 for nephrotoxic effects).
The intake of barium from food is negligible in adults at 7.5 to 9 μg/kgshort forkilogram bw/day (Health Canada, 2005), so no allocation factor is applied here. In the BfR’s opinion, a daily oral intake of 12 mgshort formilligram barium/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram.
4.1.2.5 Beryllium
Beryllium occurs in some metals and alloys in the form of impurities, but rarely as an alloy component. There is little data on the toxicity of beryllium when ingested orally; most studies have been conducted to determine its inhalative toxicity. Although the WHO (2009) attempted to derive a TDIshort forTolerable Daily Intake, the BfRshort forGerman Federal Institute for Risk Assessment considers the derivation of a TDIshort forTolerable Daily Intake for beryllium to be inappropriate due to insufficient data and is therefore unable to derive a tolerable daily intake (TDIshort forTolerable Daily Intake).
4.1.2.6 Lead
Lead is classified as toxic to reproduction (Repr. 1A) in accordance with the CLP Regulation and is therefore identified as a substance of very high concern. The EFSAshort forEuropean Food Safety Authority CONTAM Panel identifies cardiovascular effects and renal toxicity in adults and developmental neurotoxicity in children as the most critical health effects of lead (EFSAshort forEuropean Food Safety Authority CONTAM, 2010). Based on current knowledge, there is no threshold for the effects described. Therefore, EFSAshort forEuropean Food Safety Authority has not established a tolerable daily intake (TDIshort forTolerable Daily Intake) value for lead. A BMDLshort forBenchmark Dose Lower Confidence Limit01 value of 1.5 μg/kgshort forkilogram bw/day has been established for cardiovascular effects, a BMDLshort forBenchmark Dose Lower Confidence Limit10 value for renal toxicity of 0.6 μg/kgshort forkilogram bw/day and a BMDLshort forBenchmark Dose Lower Confidence Limit01 value for developmental neurotoxicity in children and adolescents (endpoint: reduction in intelligence quotient) of 0.5 μg/kgshort forkilogram bw/day derived. The BfRshort forGerman Federal Institute for Risk Assessment considers the lowest value, the BMDLshort forBenchmark Dose Lower Confidence Limit01 for developmental neurotoxicity of 0.5 μg/kgshort forkilogram bw/day, to be a suitable reference point for risk assessment.
Human exposure to lead occurs mainly through food, such as cereal products, and drinking water. In adults, the average daily intake of lead through food is 0.36 to 1.24 μg/kgshort forkilogram bw/day, and in children it is even higher, at 0.80 to 3.10 μg/kgshort forkilogram bw/day (EFSAshort forEuropean Food Safety Authority CONTAM, 2010). This dietary intake is already well above the BMDLshort forBenchmark Dose Lower Confidence Limit01 value of 0.5 μg/kgshort forkilogram bw/day derived by EFSAshort forEuropean Food Safety Authority. However, more recent figures from Germany estimateEstimateTo glossary the daily dietary lead intake of adults to be significantly lower, at 0.07 to 0.17 μg/kgshort forkilogram bw/day (Kolbaum et al.short foret alii (lat. "and others"), 2019). Nevertheless, this still results in very low margins of exposure. In general, lead intake should be as low as possible. The release of lead from food contact materials should therefore be as low as technically possible, and food contact materials should not contribute significantly to lead exposure in the opinion of the BfRshort forGerman Federal Institute for Risk Assessment. However, this contribution should not exceed 10% of the above-mentioned BMDLshort forBenchmark Dose Lower Confidence Limit01 under any circumstances. Unavoidable lead exposure from food contact materials should not exceed a daily oral intake of 3 µgshort formicrogram lead/day for a person weighing 60 kgshort forkilogram.
4.1.2.7 Cadmium
In accordance with the CLP Regulation, cadmium is classified as carcinogenic, probably toxic for reproduction and probably mutagenic (Carc. 1B, Repr. 2, Muta. 2)-. Cadmium and some of its compounds are identified as substances of very high concern and may only be used in a restricted manner under the REACH Regulation (ECHAshort forEuropean Chemicals Agency, 2016 a). EFSAshort forEuropean Food Safety Authority found no evidence that cadmium acts as a carcinogen after oral intake (EFSAshort forEuropean Food Safety Authority, 2009). The most critical effect of long-term exposure to cadmium is considered to be renal toxicity. Based on this endpoint, the EFSAshort forEuropean Food Safety Authority's CONTAM Panel derived a TWI of 2.5 μg/kgshort forkilogram bw/week for cadmium (EFSAshort forEuropean Food Safety Authority, 2009).
The main source of exposure to cadmium is food such as grain, vegetable and starchy roots (BfRshort forGerman Federal Institute for Risk Assessment, 2009). EFSAshort forEuropean Food Safety Authority estimated the average weekly dietary intake of cadmium to be 2.04 μg/kgshort forkilogram bw/week (EFSAshort forEuropean Food Safety Authority, 2012 a). As this average dietary exposure already accounts for a large proportion of the TWI, an allocation factor of 10% is applied to cadmium exposure from food contact materials. The release of cadmium from food contact materials should be as low as possible, but should not exceed a tolerable oral intake of 0.015 mgshort formilligram cadmium/week from food contact materials for a 60 kgshort forkilogram person.
4.1.2.8 Chromium
Chromium occurs naturally mainly as chromium(III). In food, which typically has a slightly acidic pH value, higher oxidised chromium compounds such as chromium(VI) compounds are unstable and decompose into chromium(III) compounds. The EFSAshort forEuropean Food Safety Authority CONTAM Panel therefore decided to consider all chromium values in food as chromium(III). It derived a TDIshort forTolerable Daily Intake of 0.3 mgshort formilligram/kgshort forkilogram bw/day for chromium from the lowest NOAEL in an animal study on chronic oral toxicity (EFSAshort forEuropean Food Safety Authority CONTAM, 2014).
The average dietary intake of chromium is estimated by the German, Austrian and Swiss Society for Nutrition to be between 61 and 84 μg/day (D-A-CH, 2019; BfRshort forGerman Federal Institute for Risk Assessment, 2021). For children, EFSAshort forEuropean Food Safety Authority estimates the average intake of chromium to be between 54.3 and 83.4 μg/day (EFSAshort forEuropean Food Safety Authority CONTAM, 2014). According to this, dietary exposure is well below the TDIshort forTolerable Daily Intake and the application of an allocation factor is not necessary. From the BfRshort forGerman Federal Institute for Risk Assessment's point of view, a daily oral intake of chromium from food contact materials should not exceed 18 mgshort formilligram/day for a person weighing 60 kgshort forkilogram.
4.1.2.9 Cobalt
Cobalt is classified in the CLP Regulation as carcinogenic, toxic to reproduction and probably mutagenic (Carc. 1B, Repr. 1B, Muta. 2) (ECHAshort forEuropean Chemicals Agency, 2016 b). In view of the lack of recent studies on the chronic toxicity of cobalt, the BfRshort forGerman Federal Institute for Risk Assessment bases its risk assessment on the conservative TDIshort forTolerable Daily Intake of the French Food Safety Agency (AFFSA). This is based on a subacute human study following oral cobalt intake, with the haematological endpoint of cobalt-induced polycythaemia (Davis et al.short foret alii (lat. "and others"), 1958). From this study, the AFFSA derived a TDIshort forTolerable Daily Intake of 1.6 μg cobalt/kgshort forkilogram bw/day (AFFSA, 2010). More recent animal data also support this value (Danzeisen et al.short foret alii (lat. "and others"), 2020). The Netherlands National Institute for Public Health and the Environment (RIVM) previously derived a TDIshort forTolerable Daily Intake of 1.4 μg/kgshort forkilogram bw/day from several studies on the consumption of alcoholic beverages containing cobalt salt, with cardiomyopathy as the endpoint (RIVM, 2001). The BfRshort forGerman Federal Institute for Risk Assessment does not follow this derivation due to various contradictions in the original literature, but instead uses the AFFSA TDIshort forTolerable Daily Intake of 1.6 μg cobalt/kgshort forkilogram bw/day (BfRshort forGerman Federal Institute for Risk Assessment, 2020).
According to EFSAshort forEuropean Food Safety Authority, the daily intake of cobalt is between 0.005 and 0.029 mgshort formilligram cobalt/day (EFSAshort forEuropean Food Safety Authority, 2012 b). Since half of the TDIshort forTolerable Daily Intake may already be exhausted by other sources, the BfRshort forGerman Federal Institute for Risk Assessment considers it justified to apply an allocation factor of 20% of the acceptable daily intake for the contribution from metal food contact materials.
For the establishment of a specific release limit (SRL) for cobalt from uncoated metal objects, the Council of Europe set an SRL of (rounded) 0.02 mgshort formilligram/kgshort forkilogram food (simulant) for cobalt in its technical guide on Metals and Alloys (EDQM, 2024). Since cobalt oxide is necessary for the production and function of enamel, DIN EN ISO 4531:2022 specifies a significantly higher SRL for cobalt of 0.1 mgshort formilligram/kgshort forkilogram food (simulant) for enamelled food contact items. Based on the (conservative) standard assumption of 60 kgshort forkilogram body weight and 1 kgshort forkilogram food consumption/day, the TDIshort forTolerable Daily Intake would thus already be exhausted. However, from the BfRshort forGerman Federal Institute for Risk Assessment's point of view, a daily oral cobalt intake of 0.02 mgshort formilligram/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram, taking into account the 20% allocation.
4.1.2.10 Iron
Iron is ubiquitous in the environment and in the human body, for example in haemoglobin as a component of red blood cells. Both an undersupply and an oversupply of iron can lead to health impairments. Chronically excessive iron intake can be associated with organ damage, for example to the liver or the intestine. This has been observed in individuals with impaired iron absorption or in people who have taken daily food supplements containing iron in the range of 100 to 1000 mgshort formilligram for over 15 years. In 2024, the EFSAshort forEuropean Food Safety Authority Panel on Nutrition, Novel Foods and Food Allergens (NDA) was unable to derive an upper limit for daily iron intake based on this data, but found that, according to studies, supplementation of up to 25 mgshort formilligram of iron per day does not lead to adverse effects in humans. Together with the background intake, EFSAshort forEuropean Food Safety Authority calculated a safe level of intake for iron at 40 mgshort formilligram/day for adults (EFSAshort forEuropean Food Safety Authority NDA 2024).
According to the National Consumption Study II, the average daily iron intake for men and women aged 14–80 is 15.2 mgshort formilligram/day and 12.3 mgshort formilligram/day, respectively (MRIshort forMax Rubner Institute, 2022). Iron is a necessary component of many metal food contact materials. Since the safe intake level for iron is thought to be well below a problematic intake level and only about one-third of this is absorbed from food, no allocation factor is used here. For a person weighing 60 kgshort forkilogram, the BfRshort forGerman Federal Institute for Risk Assessment recommends that a daily oral intake of 40 mgshort formilligram/day of iron from food contact materials should not be exceeded.
4.1.2.11 Copper
Similar to iron, copper is also an essential trace element for humans, and both a deficiency and excessive exposure to copper can have adverse health effects. Increased copper retention in the liver is considered an early indicator of potentially harmful effects from chronically excessive copper intake (EFSAshort forEuropean Food Safety Authority, 2023). EFSAshort forEuropean Food Safety Authority has set an acceptable daily intake (ADIshort forAcceptable Daily Intake) of 0.07 mgshort formilligram/kgshort forkilogram bw/day (EFSAshort forEuropean Food Safety Authority, 2023).
According to EFSAshort forEuropean Food Safety Authority, dietary copper exposure for adults is 0.015 to 0.022 mgshort formilligram/kgshort forkilogram bw/day (equivalent to 0.9 to 1.32 mgshort formilligram/day for a 60 kgshort forkilogram person) (EFSAshort forEuropean Food Safety Authority, 2023). This is consistent with previous EFSAshort forEuropean Food Safety Authority data on average copper intake in eight EU German federal states ("Laender"), which ranged from 1.27 to 1.67 mgshort formilligram/day for men and 1.15 to 1.44 mgshort formilligram/day for women (EFSAshort forEuropean Food Safety Authority, 2015). As a considerable proportion of the ADIshort forAcceptable Daily Intake is already consumed through food, the BfRshort forGerman Federal Institute for Risk Assessment considers an allocation factor of 20% to be appropriate. According to the BfRshort forGerman Federal Institute for Risk Assessment, a person weighing 60 kgshort forkilogram should not exceed a daily oral intake of 0.84 mgshort formilligram/day of copper from food contact materials.
4.1.2.12 Lithium
Since lithium is used therapeutically, most toxicological studies on lithium are based on clinical investigations of patients treated with lithium (EU-FORA, 2022). The most commonly observed adverse effects include kidney damage and hypothyroidism (McKnight et al.short foret alii (lat. "and others"), 2012). Lithium salts are used to treat mental disorders. The US Environmental Protection Agency (EPA) uses the lower limit of the therapeutic serum lithium concentration range as the LOAEL (lowest-observed-adverse-effect-level) to derive a preliminary chronic reference dose (RfD). This corresponds to an oral lithium intake of approximately 2.1 mgshort formilligram Lishort forlithium/kgshort forkilogram bw/day. With an uncertainty factor of 1000 (10 for extrapolation from a LOAEL to a NOAEL, 10 for intraspecies differences and 10 to account for inadequacies in the database), this results in an RfD of 0.002 mgshort formilligram/kgshort forkilogram bw/day (EPA, 2008). However, no TDIshort forTolerable Daily Intake for lithium could be derived from this study. Overall, the BfRshort forGerman Federal Institute for Risk Assessment has no new reliable toxicological data on lithium, so it continues to follow the TDIshort forTolerable Daily Intake of 0.008 mgshort formilligram/kgshort forkilogram bw/day derived by the RIVM in 1991 (RIVM, 1991), in line with the Council of Europe technical guide on metals and alloys.
The lithium exposure of the population through the environment and food intake can vary considerably depending on the region (Iordache et al.short foret alii (lat. "and others"), 2024). The average daily intake of lithium in adults has been estimated at 48.2 μg/day in France (ANSES, 2011), 17 µgshort formicrogram/day in England (Ysart et al.short foret alii (lat. "and others"), 1999) and 18.5 µgshort formicrogram/day in Italy (Filippini et al.short foret alii (lat. "and others"), 2020). Lithium is a necessary matrix element for the production of enamels. Since other exposure to lithium is significantly below the TDIshort forTolerable Daily Intake, it is considered acceptable for the risk assessment of exposure through food contact materials to use the TDIshort forTolerable Daily Intake without applying an allocation factor. From the BfRshort forGerman Federal Institute for Risk Assessment's point of view, a daily oral intake of lithium from food contact materials should not exceed 0.48 mgshort formilligram/day for a person weighing 60 kgshort forkilogram.
4.1.2.13 Manganese
Human and animal studies show that neurotoxicity is the most critical effect of excessive manganese intake (EFSAshort forEuropean Food Safety Authority NDA, 2023; Kern et al.short foret alii (lat. "and others"), 2010). The French ANSES uses 55 µgshort formicrogram/kgshort forkilogram bw/day as a toxicological reference value, derived from a rat study with a LOAEL of 25 mgshort formilligram/kgshort forkilogram bw/day, using neurological effects as the endpoint (ANSES, 2018; Valcke et al.short foret alii (lat. "and others"), 2018). However, according to the EFSAshort forEuropean Food Safety Authority opinion on manganese from 2023, many studies are insufficient to show a clear dose-effect relationship for manganese toxicity and to derive a tolerable upper limit for daily manganese intake. Nevertheless, EFSAshort forEuropean Food Safety Authority determined a safe level of intake at which it can be assumed with sufficient certainty that no harmful effects will occur. This value is 8 mgshort formilligram/day for adults (EFSAshort forEuropean Food Safety Authority NDA, 2023).
The main source of manganese intake is food, with grain-based foods, fruit, vegetables and nuts in particular containing high levels of manganese. According to EFSAshort forEuropean Food Safety Authority, the average daily intake of manganese is between 2.60 and 5.25 mgshort formilligram/day for men and between 2.20 and 4.69 mgshort formilligram/day for women (EFSAshort forEuropean Food Safety Authority NDA, 2023). These values are consistent with earlier studies, such as the French Total Diet Study, which found that the average exposure for adults was 2.16 mgshort formilligram/day (ANSES, 2011), and the UK Total Diet Study, which found the meanMeanTo glossary daily intake for adults to be 62 µgshort formicrogram/kgshort forkilogram bw/day (approx. 3.7 mgshort formilligram/day for 60 kgshort forkilogram bw) (FSA, 2014). Since daily intake from food already accounts for a significant proportion of the safe intake, an allocation factor of 20% is applied to intake from food contact materials. From the BfRshort forGerman Federal Institute for Risk Assessment's point of view, taking into account the 20% allocation, an oral intake of 1.6 mgshort formilligram manganese/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram.
4.1.2.14 Molybdenum
Various observational studies have linked excessive molybdenum intake to joint pain and arthritis-like symptoms, but there are no reliable chronic studies in humans that can be used for risk assessment (Hosokawa et al.short foret alii (lat. "and others"), 1994; Vyskocil et al.short foret alii (lat. "and others"), 1999). The Scientific Committee on Food (SCF) set the tolerable upper intake level (ULshort forTolerable Upper Intake Level) for molybdenum at 0.6 mgshort formilligram/day (SCF, 2000; EFSAshort forEuropean Food Safety Authority, 2024 b). This value was based on a study of reproductive toxicity in rats, in which the NOAEL was 0.9 mgshort formilligram/kgshort forkilogram bw/day (uncertainty factor: 100) (Fungwe et al.short foret alii (lat. "and others"), 1990).
According to ANSES, the average daily molybdenum intake for adults is 93.9 µgshort formicrogram/day and, according to EFSAshort forEuropean Food Safety Authority, 58 µgshort formicrogram/day in Germany (ANSES, 2011; EFSAshort forEuropean Food Safety Authority, 2013). Since a significant proportion of the ULshort forTolerable Upper Intake Level is already exhausted by other sources of exposure, an allocation factor of 20% is used for the intake of molybdenum from food contact materials. From the BfRshort forGerman Federal Institute for Risk Assessment's point of view, a daily oral intake of 0.12 mgshort formilligram molybdenum/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram, taking into account the 20% allocation.
4.1.2.15 Nickel
Elemental nickel is classified as a probable carcinogen (Carc. 2) in the CLP Regulation. A large number of soluble nickel compounds are also classified as toxic to reproduction (Repr. 1B), carcinogenic by inhalation (Carc. 1A) and probably mutagenic (Muta. 2). Nickel and its compounds are a common trigger of contact allergies. Around 15% of the German population is sensitised to nickel (BfRshort forGerman Federal Institute for Risk Assessment, 2012; Ahlström et al.short foret alii (lat. "and others"), 2019). Chronic oral exposure to nickel can lead to the occurrence of various organ damage (especially to the liver and kidneys) as well as damage to the nervous and immune systems. In its latest re-evaluation, EFSAshort forEuropean Food Safety Authority derived a TDIshort forTolerable Daily Intake of 13 µgshort formicrogram/kgshort forkilogram bw/day, which the BfRshort forGerman Federal Institute for Risk Assessment uses as the basis for its risk assessment (EFSAshort forEuropean Food Safety Authority CONTAM, 2020). Reproductive toxicity (loss of embryos after implantation) was identified as the most sensitive endpoint after chronic oral exposure in a study on rats. While the WHO derived a TDIshort forTolerable Daily Intake of 12 μg/kgshort forkilogram bw/day in its 2017 "Guidelines for Drinking-water Quality" based on a study on the triggering of allergies in already sensitised people through the intake of nickel with drinking water, it has also been following the EFSAshort forEuropean Food Safety Authority TDIshort forTolerable Daily Intake since 2022 (WHO, 2017; WHO, 2022).
The main exposure to nickel is through food. The average dietary exposure to nickel calculated by EFSAshort forEuropean Food Safety Authority is in a range between 2.90 and 3.41 µgshort formicrogram/kgshort forkilogram bw/day (0.174 mgshort formilligram/day to 0.204 mgshort formilligram/day) for adults (EFSAshort forEuropean Food Safety Authority CONTAM, 2020). In view of possible toxicological effects and the fact that a significant proportion of the TDIshort forTolerable Daily Intake is already exhausted by other sources of exposure, consumer exposure to nickel from food contact materials should be as low as possible, but should not exceed 20% of the TDIshort forTolerable Daily Intake. From the BfRshort forGerman Federal Institute for Risk Assessment's point of view, a daily oral intake of 0.156 mgshort formilligram nickel/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram, taking into account the 20% allocation.
4.1.2.16 Selenium
Selenium is an essential trace element for the human body, but chronically elevated selenium intake can cause toxic effects, which are often summarised under the collective term 'selenosis'. These include structural damage and loss of hair and nails, as well as neurological disorders (ATSDR, 2003; Fairweather-Tait et al.short foret alii (lat. "and others"), 2011). A LOAEL value for alopecia as an early sign of selenium toxicity was derived from a large randomised controlled trial in humans and is 330 μg/day (Lippman et al.short foret alii (lat. "and others"), 2009). Applying an uncertainty factor of 1.3, the EFSAshort forEuropean Food Safety Authority NDA Panel derived a tolerable upper intake level of 0.255 mgshort formilligram/day for the European population (EFSAshort forEuropean Food Safety Authority NDA, 2023).
The main source of selenium intake is food, particularly dairy and meat products, fish and cereal products. The average daily intake for adults is between 42.7 and 65.6 µgshort formicrogram/day for men and between 35.8 and 50.5 µgshort formicrogram/day for women (EFSAshort forEuropean Food Safety Authority NDA, 2023). Since daily selenium intake from food already accounts for a significant proportion of the tolerable total daily intake, an allocation factor of 20% is applied to intake from food contact materials. In the BfRshort forGerman Federal Institute for Risk Assessment's view, a daily oral intake of 0.051 mgshort formilligram selenium/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram, taking into account the 20% allocation.
4.1.2.17 Silver
As part of its biocide assessment, the ECHAshort forEuropean Chemicals Agency derived an acceptable daily intake (ADIshort forAcceptable Daily Intake) for silver of 0.9 μg/kgshort forkilogram bw/day (EFSAshort forEuropean Food Safety Authority and ECHAshort forEuropean Chemicals Agency, 2021; ECHAshort forEuropean Chemicals Agency, 2021). This was calculated from a NOAEL value of 9 mgshort formilligram silver-zinc zeolite/kgshort forkilogram bw/day (uncertainty factor 100 for inter- and intra-species differences and a further factor of 100 for conversion from silver-zinc zeolite to free silver ions), which was determined in a study on rats and is based on the pigmentation of internal organs (Takizawa et al.short foret alii (lat. "and others"), 1992). It should be noted that this ADIshort forAcceptable Daily Intake can be considered very conservative, as pigmentation of internal organs does not necessarily represent an adverse effect. In addition, the ADIshort forAcceptable Daily Intake is partly at odds with observations in humans: according to ANSES calculations, the average intake (see below) is already up to three times higher than the ADIshort forAcceptable Daily Intake without resulting in pigmentation of internal organs.
Silver can be ingested through drinking water or food, where it is also approved as a food additive. ANSES estimates the average daily intake of silver for adults to be 1.29 to 2.65 μg/kgshort forkilogram bw/day (ANSES, 2011). Formally, an allocation factor of 10% would therefore be appropriate. However, due to the very conservative ADIshort forAcceptable Daily Intake chosen as described above and the fact that the resulting intake is still considered to be very low compared to other intakes, the BfRshort forGerman Federal Institute for Risk Assessment considers an allocation factor of 20% for the intake of silver from food contact materials to be appropriate. In the BfRshort forGerman Federal Institute for Risk Assessment's view, a daily oral intake of 0.0108 mgshort formilligram silver/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram, taking into account the 20% allocation.
4.1.2.18 Thallium
Thallium is rapidly and efficiently absorbed, with excretion occurring mainly via the kidneys (EPA, 2009). Chronic exposure to thallium typically causes neurological disorders of both a sensory and motor nature, as well as hair loss (EU-FORA, 2022; Cvjetko et al.short foret alii (lat. "and others"), 2010). The concentration of thallium in urine is considered a reliable indicator of thallium exposure. Exposure to 10 µgshort formicrogram of soluble thallium compounds leads to a thallium concentration of approximately 5 µgshort formicrogram/l urine (WHO, 1996). The WHO classifies this daily intake as unlikely to have harmful effects on human health. However, it concluded that, due to uncertainties regarding the dose-effect relationship, it cannot derive a health-based limit value (WHO, 1996). The US EPA also came to this conclusion (EPA, 2009, 2012). It proposes a "provisional screening value" that could be useful in certain cases. The value is 10 ng/kgshort forkilogram bw/day and is based on a subchronic study in rats in which alopecia occurred. As the uncertainty in the data from the animal experiment is very high, it seems appropriate to rely on epidemiological data. Based on an epidemiological study, the German Federal Environment Agency (UBAshort forGerman Environment Agency) has determined a significantly lower value for oral thallium exposure of 10 μg/person/day, at which no adverse health impairments are expected (Brockhaus et al.short foret alii (lat. "and others"), 1981; UBAshort forGerman Environment Agency, 2011). The BfRshort forGerman Federal Institute for Risk Assessment recommends that the total intake of thallium from all sources should not exceed 10 µgshort formicrogram/person/day (BfRshort forGerman Federal Institute for Risk Assessment, 2004). Due to uncertainties in the underlying data, the specified value should not be regarded as a HBGVshort forHealth-Based Guidance Value. This value is used as an aid to identify an intake level that should not be exceeded due to the release of thallium from food contact materials.
Although dietary intake of thallium is low, it can be as high as 2 to 5 µgshort formicrogram/day in adults (Sherlock et al.short foret alii (lat. "and others"), 1986; FSA, 2014). Since dietary intake already accounts for a significant proportion of the maximum total intake and due to uncertainties in the data, an allocation factor of 10% is used for exposure to thallium from food contact materials. This approach is in line with the Council of Europe's recommendations on release limits for thallium from uncoated metal objects. From the BfRshort forGerman Federal Institute for Risk Assessment's point of view, taking into account the 10% allocation, a daily oral intake of 0.001 mgshort formilligram thallium/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram.
4.1.2.19 Vanadium
Vanadium is not mutagenic, but can cause numerical and structural chromosome damage. These effects are attributable to indirect mechanisms with a threshold (ATSDR, 2012; EFSAshort forEuropean Food Safety Authority, 2004). In animal studies, damage to the kidneys, spleen and lungs has been observed as a result of chronic vanadium exposure (EFSAshort forEuropean Food Safety Authority, 2004; RAC, 2020). Clinical experience in humans is limited to studies with a small number of volunteers, in which gastrointestinal disorders were observed as the most sensitive endpoint. The lowest dose of a vanadium compound reported to cause such an effect was approximately 0.2 mgshort formilligram/kgshort forkilogram bw/day (Dimond et al.short foret alii (lat. "and others"), 1963; EFSAshort forEuropean Food Safety Authority, 2004). A 1997 study involving 12 weeks of oral exposure of patients to vanadium, in which haematological and blood pressure effects were investigated, led to the determination of a NOAEL of 0.12 mgshort formilligram/kgshort forkilogram bw/day (Fawcett et al.short foret alii (lat. "and others"), 1997). This NOAEL was used by both the Agency for Toxic Substances and Disease Registry (ATSDR, 2012) and the International Council for Harmonisation (ICH) to calculate HBGVshort forHealth-Based Guidance Value (Laupheimer et al.short foret alii (lat. "and others"), 2025). The ICH derived a permitted daily exposure (PDE) of 120 μg/day (ICH, 2022). This value is supported by the results of a recent subchronic study in rats and mice, in which changes in blood count were identified as the most sensitive effect (NTP, 2023). The BfRshort forGerman Federal Institute for Risk Assessment uses this value to identify a maximum intake level that should not be exceeded through the release of vanadium from food contact materials.
Vanadium can be ingested through drinking water and food and is found in significant amounts in seafood and mushrooms, for example. Estimates of total dietary intake of vanadium in humans range from 10 to 60 μg/day (ICH, 2022). In the ANSES Total Diet Study, the average daily intake of vanadium in adults was estimated at 52 μg/day (ANSES, 2011). Since dietary intake already accounts for a significant proportion of the maximum total intake, an allocation factor of 20% is used for exposure to vanadium from food contact materials. In the BfRshort forGerman Federal Institute for Risk Assessment's view, a daily oral intake of 0.024 mgshort formilligram vanadium/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram, taking into account the 20% allocation.
4.1.2.20 Zinc
Zinc is an essential trace element and an important component of many metalloenzymes. Zinc and copper impair each other's intake, so, in the gastrointestinal tract, increased intake of one can lead to reduced intake of the other. Reduced copper absorption is considered a sensitive parameter for increased zinc absorption. This endpoint was used by the UK Expert Group on Vitamins and Minerals (EVM) and the SCF to derive a safe upper limit for zinc intake of 25 mgshort formilligram/day (EVM, 2003; SCF, 2003). This tolerable upper intake level for total daily zinc intake was reconfirmed by EFSAshort forEuropean Food Safety Authority in 2024 (EFSAshort forEuropean Food Safety Authority, 2024 b).
The main source of zinc intake is food, especially bread, meat and dairy products. According to the National Consumption Study II, the average daily zinc intake (including supplements) for men and women aged 14–80 is 12.3 mgshort formilligram/day and 9.5 mgshort formilligram/day, respectively (MRIshort forMax Rubner Institute, 2022). Since dietary intake already accounts for a substantial proportion of the safe upper limit for zinc intake, an allocation factor of 20% is used for exposure to zinc from food contact materials. From the BfRshort forGerman Federal Institute for Risk Assessment's point of view, a daily oral intake of 5 mgshort formilligram zinc/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram, taking into account the 20% allocation.
4.1.2.21 Tin
The absorption of tin and its inorganic compounds is very low. Oral intake of high amounts of tin can lead to gastrointestinal complaints, which is why there are legal maximum levels (Regulation (EU) 2023/915) for tin concentrations in canned food and canned beverages of 200 and 100 mgshort formilligram/kgshort forkilogram, respectively. According to the WHO, there is no evidence of chronic tin toxicity in humans, so it was not considered necessary to set a guideline value for drinking water (WHO, 2022). The oral toxicity of tin was investigated in a 28-day study on rats back in 2010, and no harmful effects were found even at the highest daily dose of 1000 mgshort formilligram/kgshort forkilogram bw (ECHAshort forEuropean Chemicals Agency, 2023). However, as tin was administered in powder form, this study is not representative of human exposure via food, where tin is usually present in ionic form. In a subchronic study conducted in 1973 with tin(II) chloride in rats, effects such as growth inhibition, reduced food efficiency, mild anaemia and histological changes in the liver were observed at concentrations of 0.3% tin chloride in the feed. The NOAEL was converted (cf. EFSAshort forEuropean Food Safety Authority, 2012 c) to 81 mgshort formilligram/kgshort forkilogram bw/day (de Groot et al.short foret alii (lat. "and others"), 1973).
The RIVM derived a TDIshort forTolerable Daily Intake for chronic tin exposure from a NOAEL value in a study on rats (RIVM, 2009). The slight increase in tin accumulation in the bones and the decrease in feed efficiency were identified as the most sensitive endpoints. The TDIshort forTolerable Daily Intake of 0.2 mgshort formilligram/kgshort forkilogram bw/day was derived from the NOAEL of 20 mgshort formilligram/kgshort forkilogram bw/day and the application of an uncertainty factor of 100 (10 for inter- and 10 for intra-species variation). This TDIshort forTolerable Daily Intake is also used here for the risk assessment.
ANSES estimates the average daily dietary intake of tin for adults to be 3.9 μg/kgshort forkilogram bw/day (ANSES, 2011). According to this, dietary exposure is well below the TDIshort forTolerable Daily Intake and the application of an allocation factor is not necessary. In the BfRshort forGerman Federal Institute for Risk Assessment's view, a daily oral intake of 12 mgshort formilligram tin/day from food contact materials should not be exceeded for a person weighing 60 kgshort forkilogram.
4.1.2.22 Summary and health-based guidance values (HBGVshort forHealth-Based Guidance Value)
Based on existing toxicological studies and corresponding assessments by other authorities, the BfRshort forGerman Federal Institute for Risk Assessment has derived HBGVshort forHealth-Based Guidance Value or toxicological reference values for 20 of the 21 elements examined. In addition, allocation factors were defined where necessary to describe the maximum proportion of the respective HBGVshort forHealth-Based Guidance Value or toxicological reference values that can be utilised through exposure via food contact materials from the BfRshort forGerman Federal Institute for Risk Assessment's perspective. Under the assumption of a person with a body weight of 60 kgshort forkilogram, daily and weekly maximum tolerable intake levels were then calculated. The results are summarised in Table 1 and 2.
Table 1
Health-based guidance values (HBGVshort forHealth-Based Guidance Value) and derived maximum tolerable intake levels for the 17 elements investigated. The BfRshort forGerman Federal Institute for Risk Assessment recommends that the exposure contributions from food contact materials (FCM) listed in the last column should not be exceeded.
| Element | Type of HBGV | Source | HBGV in mg/kg bw/day or *week | Maximum tolerable intake in mg per person (60 kg) and day or *week | Allocation factor (AF) | Recommended maximum intake from FCM in mg/person/day or *week |
|---|---|---|---|---|---|---|
| Aluminium | TWI | EFSA (2008) | *1 | *60 | 10% | *6 |
| Antimony | TDI | WHO (2003) | 0.006 | 0.36 | - | |
| Barium | TDI | SCHER (2012) | 0.2 | 12 | - | |
| Cadmium | TWI | EFSA CONTAM (2012) | *0.0025 | *0.15 | 10% | *0.015 |
| Chromium | TDI | EFSA CONTAM (2014) | 0.3 | 18 | - | |
| Cobalt | TDI | EFSA (2012) | 0.0016 | 0.1 | 20% | 0.02 |
| Iron | Safe level of intake | EFSA NDA (2024) | - | 40 | - | |
| Copper | ADI | EFSA (2022) | 0.07 | 4.2 | 20% | 0.84 |
| Lithium | TDI | RIVM (1991) | 0.008 | 0.48 | - | |
| Manganese | Safe level of intake | EFSA NDA (2023) | - | 8 | 20% | 1.6 |
| Molybdenum | UL | SCF (2006) | - | 0.6 | 20% | 0.12 |
| Nickel | TDI | EFSA CONTAM (2020) | 0.013 | 0.78 | 20% | 0.156 |
| Selenium | UL | EFSA NDA (2023) | - | 0.255 | 20% | 0.051 |
| Silver | ADI | ECHA (2021) | 0.0009 | 0.054 | 20% | 0.0108 |
| Vanadium | PDE | ICH (2022) | 0.002 | 0.12 | 20% | 0.024 |
| Zinc | UL | SCF (2003) | - | 25 | 20% | 5 |
| Tin | TDI | RIVM (2009) | 0.2 | 12 | - |
Table 2
Toxicological reference or guidance values used for guidance for the elements investigated for which no HBGVshort forHealth-Based Guidance Value could be derived. In the BFR’s opinion, the release of these elements from food contact materials (FCMs) should be as low as possible and should not contribute significantly to exposure. For unavoidable exposure from FCM, the maximum daily intake levels specified in the table should not be exceeded. Due to insufficient data, neither an HBGVshort forHealth-Based Guidance Value nor a toxicological reference value could be derived for beryllium.
| Element | Toxico-logical reference value | Source | Toxicological reference value in mg/kg BW/day | Toxicological reference value in mg per person (60 kg) per day | Allocation factor (AF) | Maximum unavoidable exposure via FCM in mg/person/day | |
|---|---|---|---|---|---|---|---|
| Arsenic | BMDL05 | EFSA (2024) | 0.00006 | 0.0036 | 10% | 0.00036 | |
| Beryllium | - | - | - | - | |||
| Lead | BMDL01 | EFSA (2010) | 0.0005 | 0.03 | 10% | 0.003 | |
| Thallium | Maximum exposure | WHO (1996) | - | 0.01 | 10% | 0.001 | |
4.1.3 Element release and technical assessment
For the following exposure assessment, the BfRshort forGerman Federal Institute for Risk Assessment uses the available monitoring data from the regional authorities of the German federal states ("Laender") on the release of elements from metal objects intended to come into contact with food from 2022. Release tests were carried out in food simulants under various test conditions.
The summarised results of the release tests and the specific release limit values (SRLs) of the Council of Europe and DIN EN ISO 4531:2022 are shown in Table 3. These limit values serve as compliance criteria for the technical suitability assessment of food contact materials. The measured element releases were also compared with these limit values. For the risk assessment, however, the derived HBGVshort forHealth-Based Guidance Value or toxicological reference values are used.
The release tests for uncoated items were carried out in artificial tap water and in 0.5% citric acid (at 40 °Cshort fordegrees Celsius/70 °Cshort fordegrees Celsius/100 °Cshort fordegrees Celsius), based on the prescribed test conditions of the Council of Europe recommendations. Based on the DIN EN ISO 4531:2022 standard, enamelled objects were tested in 3% acetic acid in hot contact for 2 hours (70 °Cshort fordegrees Celsius or 95 °Cshort fordegrees Celsius) and in 4% acetic acid at room temperature for 24 hours. The temperature was selected according to the type of use of the respective object. For example, an uncoated saucepan was tested at 100 °Cshort fordegrees Celsius, a coffee spoon at 70 °Cshort fordegrees Celsius, and an apple slicer at 40 °Cshort fordegrees Celsius. In the case of enamelled items, for example, a dessert plate was tested at 70 °Cshort fordegrees Celsius and an oven dish at 95 °Cshort fordegrees Celsius. To reflect repeated use, three consecutive migrations were carried out and the results of the third migration were used for assessment.
A total of 194 items were examined, 115 of which were uncoated and 79 enamelled. The elemental release of 21 different elements was examined. The elements copper, cobalt and zinc were examined in all samples. The elements aluminium, antimony, barium, cadmium, lead, chromium, iron, lithium and nickel were examined in the vast majority (over 75%) of the samples, and the elements arsenic, beryllium, manganese, molybdenum, selenium, silver, thallium, vanadium and tin were examined in less than 75% of the samples. The elements tested were determined by the analytical equipment available in the testing laboratories.
As described above, release tests were carried out for each element in two food simulants. For the following evaluations, the higher measured release value was used in each case to ensure a conservative, "worst-case" estimate of element release.
The majority of the samples tested showed release values below the respective SRLs. 99.1% of the uncoated samples demonstrated compliance with all SRLs, while this was only true for 53.2% of the enamelled samples. Overall, at least one SRL value was exceeded in 38 of the 194 samples analysed, with 37 of these 38 samples being enamelled. In 24 of the 38 samples, the release of a single element exceeded the respective release limit value, and in 14 samples, the release of several elements exceeded the respective release limit value. Aluminium is the element that shows release quantities above the SRL in most samples. In the new version of DIN EN ISO 4531:2022, the limit value for aluminium was lowered to 1 mgshort formilligram/kgshort forkilogram food simulant; in the previous version from 2018, the SRL for aluminium was still 5 mgshort formilligram/kgshort forkilogram food simulant. Accordingly, the new limit value of 1 mgshort formilligram/kgshort forkilogram was exceeded in 33 samples, compared to 2 samples that would have exceeded the previous SRL of 5 mgshort formilligram/kgshort forkilogram.
Table 3
Element release quantities in mgshort formilligram/kgshort forkilogram simulant from the uncoated and enamelled kitchen items examined and comparison with the specific release limits (SRL) according to Council of Europe technical Guide on metals and alloys in food contact for uncoated metal items a and according to standard DIN EN ISO 4531:2022 for enamelled metal items b.
| Element | Sample type | Number of samples | Mean value | Median | Maximum | 90th percentile | SRL | Samples > SRL |
|---|---|---|---|---|---|---|---|---|
| Aluminium | uncoated | 115 | 0.0305 | 0.012 | 0.681 | 0.05 | 5 | 0 |
| enamelled | 73 | 1.86 | 0.871 | 43.0 | 3.426 | 1 | 33 | |
Antimony
| uncoated | 110 | 0.000818 | 0.0005 | 0.00318 | 0.0015 | 0.04 | 0 |
| enamelled | 70 | 0.00609 | 0.0015 | 0.077 | 0.0138 | 0.04 | 1 | |
| Arsenic | uncoated | 92 | 0.000253 | 0.00015 | 0.00158 | 0.0005 | 0.002 | 0 |
| enamelled | 46 | 0.000347 | 0.000379 | 0.002 | 0.0005 | 0.002 | 0 | |
| Barium | uncoated | 100 | 0.0139 | 0.009 | 0.033 | 0.033 | 1.2 | 0 |
| enamelled | 79 | 0.685 | 0.025 | 18.3 | 1.05 | 1.2 | 6 | |
| Beryllium | uncoated | 37 | 0.000755 | 0.0003 | 0.003 | 0.0025 | 0.01 | 0 |
| enamelled | 25 | 0.00021 | 0.0003 | 0.0003 | 0.0003 | / | 0 | |
| Lead | uncoated | 104 | 0.000417 | 0.0003 | 0.00573 | 0.001 | 0.01 | 0 |
| enamelled | 70 | 0.0760 | 0.000539 | 4.62 | 0.0146 | 0.01 | 7 | |
| Cadmium | uncoated | 111 | 0.000219 | 0.00015 | 0.0044 | 0.0005 | 0.005 | 0 |
| enamelled | 62 | 0.0123 | 0.000247 | 0.721 | 0.00190 | 0.005 | 3 | |
| Chromium | uncoated | 115 | 0.0162 | 0.005 | 0.348 | 0.0399 | 1 | 0 |
| enamelled | 74 | 0.114 | 0.0015 | 7.74 | 0.0323 | 1 | 1 | |
| Cobalt | uncoated | 115 | 0.00227 | 0.00045 | 0.143 | 0.0025 | 0.02 | 1 |
| enamelled | 79 | 0.0552 | 0.00355 | 1.55 | 0.0854 | 0.1 | 5 | |
| Iron | uncoated | 100 | 25.5 | 0.15 | 2490 | 1.10 | 40 | 1 |
| enamelled | 77 | 157 | 0.050 | 12000 | 0.50 | / | 0 | |
| Copper | uncoated | 115 | 0.0306 | 0.0055 | 0.15 | 0.15 | 4 | 0 |
| enamelled | 79 | 0.107 | 0.016 | 0.888 | 0.50 | 4 | 0 | |
| Lithium | uncoated | 107 | 0.00151 | 0.00075 | 0.013 | 0.005 | 0.048 | 0 |
| enamelled | 69 | 0.0824 | 0.0085 | 2.37 | 0.0973 | 0.48 | 4 | |
| Manganese | uncoated | 72 | 0.105 | 0.0125 | 4.9 | 0.114 | 0.55 | 1 |
| enamelled | 56 | 1.65 | 0.025 | 84.8 | 0.5 | 0.55 | 2 | |
| Molybdenum | uncoated | 52 | 0.00153 | 0.0005 | 0.0133 | 0.00485 | 0.12 | 0 |
| enamelled | 35 | 0.00130 | 0.00015 | 0.009 | 0.004 | 0.12 | 0 | |
| Nickel | uncoated | 112 | 0.00282 | 0.00105 | 0.032 | 0.00556 | 0.14 | 0 |
| enamelled | 66 | 0.0176 | 0.00199 | 0.507 | 0.0361 | 0.14 | 2 | |
| Selenium | uncoated | 23 | 0.00294 | 0.00375 | 0.00375 | 0.00375 | / | 0 |
| enamelled | 30 | 0.00244 | 0.0015 | 0.0104 | 0.005 | / | 0 | |
| Silver | uncoated | 19 | 0.00301 | 0.0025 | 0.006 | 0.0055 | 0.08 | 0 |
| enamelled | 17 | 0.000804 | 0.000487 | 0.0015 | 0.0015 | 0.08 | 0 | |
| Thallium | uncoated | 54 | 0.000053 | 0.000025 | 0.00015 | 0.00015 | 0.001 | 0 |
| enamelled | 39 | 0.000204 | 0.000025 | 0.0005 | 0.0005 | / | 0 | |
| Vanadium | uncoated | 52 | 0.00142 | 0.0009 | 0.02 | 0.0025 | 0.01 | 1 |
| enamelled | 35 | 0.0128 | 0.00075 | 0.413 | 0.00194 | 0.01 | 1 | |
| Zinc | uncoated | 115 | 0.0559 | 0.0225 | 2.29 | 0.150 | 5 | 0 |
| enamelled | 79 | 0.110 | 0.035 | 1.23 | 0.50 | 5 | 0 | |
| Tin | uncoated | 42 | 0.00794 | 0.003 | 0.03 | 0.0240 | 100 | 0 |
| enamelled | 32 | 0.00231 | 0.00015 | 0.0279 | 0.0105 | / | 0 |
a Food simulants used: artificial tap water and 0.5% citric acid, depending on the type of use of the item, tested at 40 °Cshort fordegrees Celsius/70 °Cshort fordegrees Celsius/100 °Cshort fordegrees Celsius for 30 minutes or 2 hours. The higher release value was used for each item.
b Food simulants used: 3% acetic acid in hot contact for 2 hours (70 °Cshort fordegrees Celsius or 95 °Cshort fordegrees Celsius) and in 4% acetic acid at room temperature for 24 hours. The higher release value was used for each item.
Overall, it can be seen that – apart from aluminium release from enamelled samples – the technical requirements are met by the vast majority of the items examined for all elements. In the case of individual items, some of which significantly exceed one or more SRLs, the BfRshort forGerman Federal Institute for Risk Assessment believes that manufacturers should review the quality of their raw materials and manufacturing processes or exclude the use of these products, particularly for hot acidic food.
4.1.4 Exposure assessment and risk characterisation
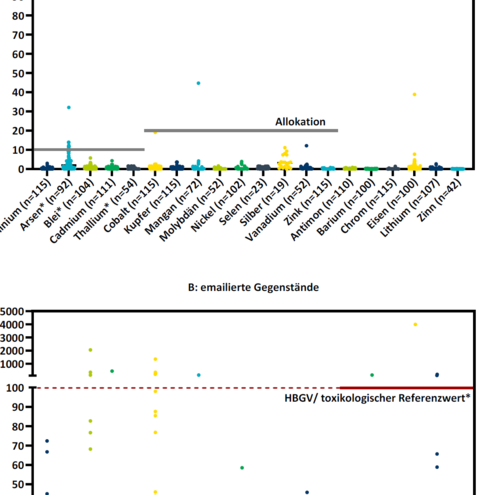
n order to estimate the actual exposure to the elements examined through food consumed that has come into contact with metal kitchenware, the following consumption quantities were assumed: For items with large capacities of more than 1 litre (cooking pots, roasting pans, baking trays, etc.), it was assumed that an adult consumes 1 kgshort forkilogram of food per day that has come into contact with these items. For items with smaller capacities (cups, cocktail shakers) or enveloping volumes (cutlery, whisks, pizza cutters, etc.) than 1 litre, it was assumed that the total volume of food simulant used for the test corresponds to the daily consumption amount, as the food that comes into contact with these items in the household usually has a significantly smaller volume than 1 litre. The daily or weekly intake for the respective elements was then compared with the HBGVshort forHealth-Based Guidance Value or toxicological reference values for a 60 kgshort forkilogram adult specified in section 4.1.2 (see alsoTable 1). Where deemed necessary, an allocation factor (Table 1) was also taken into account. The results are shown in Figure 1.
Calculation example 1:
An enamelled pot showed aluminium release in 3% acetic acid (2 hours at 95 °Cshort fordegrees Celsius) and 4% acetic acid (24 hours at 22 °Cshort fordegrees Celsius) of 3.04 and 8.27 mgshort formilligram/kgshort forkilogram simulant, respectively, in the third migration test. The simulant volume was 750 mlshort formillilitre. The higher release value from the determination in 4% acetic acid for 24 hours at 22 °Cshort fordegrees Celsius was used for the calculation. Based on the assumptions described above, the following weekly exposure was obtained:
\mathit{Exposition} = 8,27 \frac{\mathit{mg}}{\mathit{kg}} * 0,75 \frac{\mathit{kg}}{\mathit{Tag}} / 60 \mathit{ kg} \mathit{ KG} * 7 \frac{\mathit{Tage}}{\mathit{Woc} h e} = 0,724 \frac{\mathit{mg}}{\mathit{ kg} \mathit{ KG} * \mathit{Woc} h e}
These values correspond to an exceedance of the SRL for aluminium from enamel (1 mgshort formilligram/kgshort forkilogram) and the allocated 10% of the tolerable weekly intake (TWI) for aluminium (HBGVshort forHealth-Based Guidance Value = 1 mgshort formilligram/kgshort forkilogram BW/week) – but not an exceedance of the HBGVshort forHealth-Based Guidance Value itself.
Calculation example 2:
An enamelled baking tray showed a cobalt release in 3% acetic acid (2 hours at 95 °Cshort fordegrees Celsius) and 4% acetic acid (24 hours at 22 °Cshort fordegrees Celsius) of 0.35 mgshort formilligram/kgshort forkilogram simulant or < limit of quantification (0.004 mgshort formilligram/kgshort forkilogram simulant). The simulant volume was 1410 mlshort formillilitre. The higher release value from the determination in 3% acetic acid for 2 hours at 95 °Cshort fordegrees Celsius was used for the calculation. Based on the assumptions described above, the following daily exposure was calculated:
\mathit{Exposition} = 0,35 \frac{\mathit{mg}}{\mathit{kg}} * 1 \frac{\mathit{kg}}{\mathit{Tag}} / 60 \mathit{kg} \mathit{KG} = 0,0058 \frac{\mathit{mg}}{\mathit{kg} \mathit{KG} * \mathit{Tag}}
These values result in both an exceedance of the SRL for cobalt (0.1 mgshort formilligram/kgshort forkilogram) and the tolerable daily intake (TDIshort forTolerable Daily Intake) for cobalt (HBGVshort forHealth-Based Guidance Value = 0.0016 mgshort formilligram/kgshort forkilogram BW/day, 0.1 mgshort formilligram/day for a person weighing 60 kgshort forkilogram).
Overall, the release of elements from the vast majority of the samples tested does not result in the derived HBGVshort forHealth-Based Guidance Value being exceeded, even when allocation factors are taken into account. The calculated margins of exposure were (far) greater than 1 for arsenic in all samples and for lead in the vast majority of samples. The calculated exposure exceeds one or more HBGVs in a total of 8 (out of 194) samples or corresponds to a margin of exposure for lead of less than 1. These 8 samples consist of seven enamelled kitchen items and one without coating. In the BfR’s opinion, these items are not suitable for contact with food.
Cobalt is a special case. The release of cobalt from 5 samples (4 of which are enamelled) leads to an exceedance of the HBGVshort forHealth-Based Guidance Value of 0.1 mgshort formilligram/day (for a person weighing 60 kgshort forkilogram). In the BfR’s view, these items are not suitable for daily use in contact with food. The release of cobalt from a further 6 samples resulted in the allocated HBGVshort forHealth-Based Guidance Value of 0.02 mgshort formilligram/day being exceeded, but not in the HBGVshort forHealth-Based Guidance Value itself being exceeded. From a toxicological point of view, the contribution of these items to the overall exposure to cobalt is assessed as too high. However, cobalt oxide cannot be entirely omitted as an adhesion promoter for the manufacture and function of enamelled surfaces. The ALARA principle ("As Low As Reasonably Achievable") applies accordingly to the use of cobalt in food contact materials made of enamel. The large number of enamelled items whose cobalt release did not result in the exceedance of allocated HBGVshort forHealth-Based Guidance Value for cobalt shows that this is technically possible.
Taking into account the allocations of 10-20% for elements that are also subject to significant exposure from other sources, 39 samples exceeded the allocated HBGVshort forHealth-Based Guidance Value or the value considered to be the maximum exposure contribution for lead (0.003 mgshort formilligram/person/day) or arsenic (0.00036 mgshort formilligram/person/day) even in the case of unavoidable release. The elements for which exceedances occurred in most samples are aluminium, arsenic, lead and cobalt. In 28 of 39 samples, exceedances occurred for a single element, while in 11 samples (5.7% of all samples), several elements were affected. These 11 samples consist of ten enamelled kitchen items and one without coating. As the use of these items contributes significantly to the overall intake of some elements, the BfRshort forGerman Federal Institute for Risk Assessment believes that manufacturers should revise their raw material qualities and manufacturing processes so that exposure from these food contact materials is below the allocated HBGVshort forHealth-Based Guidance Value for all elements and below an exposure contribution of 0.003 mgshort formilligram lead/person/day and 0.00036 mgshort formilligram arsenic/person/day.
As expected, the occurrence of increased element release tends to occur when testing in acidic simulants and at elevated temperatures – i.e. for items such as pots, roasting pans and oven trays. For elements for which the SRL value was frequently exceeded, there is also a tendency for the respective HBGVshort forHealth-Based Guidance Value/toxicological reference value or the allocated share thereof to be exceeded more frequently.
In summary, the risk assessment based on the exposure assessment showed that the vast majority of enamelled or uncoated metal food contact materials are suitable for food contact. However, with regard to overall exposure, taking into account possible additional sources of intake (such as food), the BfRshort forGerman Federal Institute for Risk Assessment considers that some items contribute too much to the daily intake of certain elements. The BfRshort forGerman Federal Institute for Risk Assessment recommends that manufacturers of these items review their raw materials and manufacturing processes in order to further reduce element release. Only a few objects showed element releases that could exceed the derived HBGVshort forHealth-Based Guidance Value or toxicological reference values and thus increase the risk of the occurrence of health impairments. From a toxicological point of view, these objects are not suitable for contact with food.
Figure 1
4.1.5 Consideration of uncertainties
In the present assessment, there is uncertainty both when it comes to the toxicological derivation of HBGVshort forHealth-Based Guidance Value or toxicological reference value and in the analytical determination of element release and exposure assessment.
In terms of toxicology, uncertainties are mainly due to incomplete data or low-quality studies. An attempt was made to address these uncertainties by reviewing the studies for their suitability and selecting the study with the lowest NOAEL or BMDLshort forBenchmark Dose Lower Confidence Limit from all suitable studies for each element, as well as by using appropriate assessment and uncertainty factors. Experience has shown that this approach usually results in a conservative HBGVshort forHealth-Based Guidance Value or toxicological reference values.
Food simulants and test conditions designed to describe the most unfavourable realistic use case were used to determine element releases. Conversely, this also means that there are uses (i.e. certain food, contact times and temperatures) in which significantly lower amounts of elements are transferred than in the tests carried out. Given the variety of uses for most kitchen items, this tends to lead to an overestimation of the average regular release quantities.
Conservative assumptions were used to estimate exposure from element releases from individual items. Contrary to these assumptions, however, it can be assumed that only a few of the items examined are used daily and throughout a person's lifetime. Therefore, the actual exposure from a particular item is generally lower than the values calculated here. A good picture of exposure can be obtained by looking at the total number of items examined.
An overall assessment of the uncertainties shows that the present assessment was conducted conservatively and, in particular, that the actual exposure from the use of the examined items was likely overestimated rather than underestimated. However, individual items with particularly high element release can contribute substantially to exposure even if they are not used on a daily basis.
5 References
AFSSA (2010): French Agency for Food Safety (AFSSA). 2010. Opinion of the French Food Safety Agency on a request for scientific and technical support regarding the migration of cobalt from porcelain oven dishes intended to come into contact with food. AFSSA – Request no. 2010-SA-0095. www.anses.fr/en/system/files/MCDA2010sa0095EN.pdf
Ahlström et al.short foret alii (lat. "and others") (2019): Ahlström M.G., Thyssen J.Pshort forphosphorus., Wennervaldt M., Menné T., Johansen J.D. 2019. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis. 81, 227–241. DOI: 10.1111/cod.13327
ANSES (2011): Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail (ANSES). 2011. Second French Total Diet Study (TDS 2) – Report 1 – Inorganic contaminants, minerals, persistent organic pollutants, mycotoxins and phytoestrogens. ANSES. www.anses.fr/en/system/files/PASER2006sa0361Ra1EN.pdf
ANSES (2018): French Agency for Food, Environmental and Occupational Health & Safety (ANSES). 2018. Opinion related to the determination of manganese maximal safety value allowed in drinking water. ANSES Report No. EAUX2016SA0203, www.anses.fr/fr/system/files/EAUX2016SA0203.pdf
ATSDR (2003): Agency for Toxic Substances and Disease Registry (ATSDR). 2013. Toxicological profile for selenium. U.S. Department of Health and Human Services, Public Health Service.
ATSDR (2007): Agency for Toxic Substances and Disease Registry (ATSDR). 2007. Toxicological profile for barium and barium compounds. U.S. Department of Health and Human Services Public Health Service.
ATSDR (2012): Agency for Toxic Substances and Disease Registry (ATSDR). 2012. Toxicological profile for vanadium. U.S. Department of Health and Human Services Public Health Service.
BfRshort forGerman Federal Institute for Risk Assessment (2004): German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment). 2004. Thallium in natural mineral water. Updated opinion 003/2006 of the BfRshort forGerman Federal Institute for Risk Assessment dated 14 December 2004. www.thebfr.bund.de/cm/343/thallium_in_natuerlichem_mineralwasser.pdf
BfRshort forGerman Federal Institute for Risk Assessment (2009): German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment). 2009. Cadmium in food. BfRshort forGerman Federal Institute for Risk Assessment Press Office. ISBN: 3-938163-50-X. www.bfr.bund.de/cm/350/cadmium_in_lebensmitteln.pdf
BfRshort forGerman Federal Institute for Risk Assessment (2012): German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment). 2012. Nickel in tattooing agents can cause allergies. BfRshort forGerman Federal Institute for Risk Assessment Opinion No. 012/2013 of 25 October 2012. www.thebfr.bund.de/cm/343/nickel-in-taetowiermitteln-kann-allergien-ausloesen.pdf
BfRshort forGerman Federal Institute for Risk Assessment (2015): German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment). 2015. Arsenic in rice and rice products – Opinion No. 018/2015 of 24 June 2015. www.thebfr.bund.de/cm/343/arsen-in-reis-und-reisprodukten.pdf
BfRshort forGerman Federal Institute for Risk Assessment (2019): German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment). 2019. Reducing aluminium intake can minimise potential health risks. Opinion No. 045/2019 of the BfRshort forGerman Federal Institute for Risk Assessment dated 18 November 2019. DOI: 10.17590/20191115-135258
BfRshort forGerman Federal Institute for Risk Assessment (2020): German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment). 2020. Ceramic tableware: BfRshort forGerman Federal Institute for Risk Assessment recommends lower release levels for lead and cadmium. Opinion No. 043/2020 of 21 September 2020. DOI: 10.17590/20200921-112429
BfRshort forGerman Federal Institute for Risk Assessment (2021): German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment). 2021. Proposed maximum levels for chromium in food, including food supplements. www.bfr.bund.de/cm/343/hoechstmengenvorschlaege-fuer-chrom-in-food-inklusive-nahrungsergaenzungsmitteln.pdf
BfRshort forGerman Federal Institute for Risk Assessment (2023): German Federal Institute for Risk Assessment (BfRshort forGerman Federal Institute for Risk Assessment). 2023. New studies on aluminium-containing antiperspirants: Health impairments from aluminium intake through the skin are unlikely. Opinion 045/2023 of 6 October 2023. DOI: 10.17590/20231006-150131-0
Brockhaus et al.short foret alii (lat. "and others") (1981): Brockhaus A., Dolgner R., Ewers U., Krämer U., Soddemann H., Wiegand H. 1981. Intake and health effects of thallium among a population living in the vicinity of a cement plant emitting thallium-containing dust. International Archives of Occupational and Environmental Health. 48(4), 375-89. DOI: 10.1007/BF00378686
Cvjetko et al.short foret alii (lat. "and others") (2010): Cvjetko Pshort forphosphorus., Cvjetko I., Pavlica M. 2010. Thallium toxicity in humans. Archives of Industrial Hygiene and Toxicology. 61(1), 111-9. DOI: 10.2478/10004-1254-61-2010-1976
COT (2013): Committee on toxicity of chemicals in food, consumer products and the environment (COT). 2013. Statement on the potential risks from aluminium in the infant diet. COT Statement 2013/01. cot.food.gov.uk/sites/default/files/cot/statealuminium.pdf
D-A-CH (2019). German Nutrition Society, Austrian Nutrition Society, Swiss Nutrition Society (D-A-CH). 2019. Dietary reference value (DRV) for nutrient intake. Bonn, 2nd edition, 5th updated edition, 2019
Danzeisen et al.short foret alii (lat. "and others") (2020): Danzeisen R., Williams D.L., Viegas V., Dourson M., Verberckmoes S., Burzlaff A. 2020. Bioelution, Bioavailability, and Toxicity of Cobalt Compounds Correlate. Toxicological Sciences. 174 (2), 311-325. DOI: 10.1093/toxsci/kfz249
Davis et al.short foret alii (lat. "and others") (1958): Davis, J., Fields J. 1958. Experimental Production of Polycythemia in Humans by Administration of Cobalt Chloride. Proceedings of the Society for Experimental Biology and Medicine. 99 (2), 493-495. DOI: 10.3181/00379727-99-24395
De Groot et al.short foret alii (lat. "and others") (1973): de Groot A.Pshort forphosphorus., Feron V.J., Til H.Pshort forphosphorus. 1973. Short-term toxicity studies on some salts and oxides of tin in rats. Food and Cosmetics Toxicology. 11/1, 19-30. DOI: 10.1016/0015-6264(73)90058-8
Dimond et al.short foret alii (lat. "and others") (1963): Dimond E.G., Caravaca J., Benchimol A. 1963. Excretion, toxicity, lipid effect in man. The American Journal of Clinical Nutrition. 12, 49-53. DOI: 10.1093/ajcn/12.1.49
DIN EN ISO (2022): DIN Standards Committee for Materials Testing. 2022. DIN EN ISO 4531:2022; Enamels – Release from enamelled articles intended to come into contact with food – Test methods and permissible levels (ISO 4531:2022), German version EN ISO 4531:2022
ECHAshort forEuropean Chemicals Agency (2016 a): European Chemicals Agency (ECHAshort forEuropean Chemicals Agency). 2016. ANNEX XVII TO REACH – Conditions of restriction, Restrictions on the manufacture, placing on the market and use of certain dangerous substances, mixtures and articles, Entry 23: Cadmium. echa.europa.eu/documents/10162/3bfef8a3-8c97-4d85-ae0b-ac6827de49a9
ECHAshort forEuropean Chemicals Agency (2016 b): European Chemicals Agency (ECHAshort forEuropean Chemicals Agency). 2016. Proposal for Harmonised Classification and Labelling - Substance Name: Cobalt. Dossier submitter: RIVM/SEC, Bureau REACH. echa.europa.eu/documents/10162/d1ca0305-88d5-5b07-69ee-1f4312c1951f; Summary of Classification and Labelling for Cobalt: echa.europa.eu/de/information-on-chemicals/cl-inventory-database/-/discli/details/34808
ECHAshort forEuropean Chemicals Agency (2021): European Chemicals Agency (ECHAshort forEuropean Chemicals Agency). 2012. Competent Authority Reports, Evaluation of active substances: Silver zinc zeolite. Evaluating Competent Authority: Swedish Chemicals Agency. echa.europa.eu/documents/10162/af925783-8a82-25d7-1bfd-ae6ee15b4ee7
ECHAshort forEuropean Chemicals Agency (2023): European Chemicals Agency (ECHAshort forEuropean Chemicals Agency). 2023. Registered substances factsheet for Tin (CAS No 7440-31-5). echa.europa.eu/de/registration-dossier/-/registered-dossier/15457/7/6/2; last accessed on 26 March 2025
EDQM (2024): European Directorate for Quality of Medicines & Healthcare (EDQM). 2024. Metals and alloys used in food contact materials and articles. Council of Europe, European Directorate for Quality of Medicines & Healthcare. ISBN 978-92-871-9436-7
EFSAshort forEuropean Food Safety Authority (2004): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2004. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Vanadium. The EFSAshort forEuropean Food Safety Authority Journal. 33, 1-22. DOI: 10.2903/j.efsa.2004.33
EFSAshort forEuropean Food Safety Authority (2009): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2009. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on cadmium in food. The EFSAshort forEuropean Food Safety Authority Journal. 980, 1–139. DOI: 10.2903/j.efsa.2009.980
EFSAshort forEuropean Food Safety Authority (2012 a): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2012. Scientific report of EFSAshort forEuropean Food Safety Authority - Cadmium dietary exposure in the European population. EFSAshort forEuropean Food Safety Authority Journal. 10 (1), 2551. DOI: 10.2903/j.efsa.2012.2551
EFSAshort forEuropean Food Safety Authority (2012 b): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2012. Scientific Opinion on safety and efficacyPositive Predictive ValueTo glossary of cobalt compounds (E3) as feed additives for all animal species: Cobaltous acetate tetrahydrate, basic cobaltous carbonate monohydrate and cobaltous sulphate heptahydrate, based on a dossier submitted by TREAC EEIG. EFSAshort forEuropean Food Safety Authority Journal. 10(7), 2791. DOI: 10.2903/j.efsa.2012.2791
EFSAshort forEuropean Food Safety Authority (2012 c): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2012. Guidance on selected default values to be used by the EFSAshort forEuropean Food Safety Authority Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSAshort forEuropean Food Safety Authority Journal. 10(3), 2579. DOI: 10.2903/j.efsa.2012.2579
EFSAshort forEuropean Food Safety Authority (2013): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2013. Scientific Opinion on Dietary Reference Values for molybdenum. EFSAshort forEuropean Food Safety Authority Journal. 11(8), 3333. DOI: 10.2903/j.efsa.2013.3333
EFSAshort forEuropean Food Safety Authority (2015): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2015. Scientific Opinion on Dietary Reference Values for copper. NDA. EFSAshort forEuropean Food Safety Authority Journal. 13(10), 4253. 10.2903/j.efsa.2015.4253.
EFSAshort forEuropean Food Safety Authority (2021): EFSAshort forEuropean Food Safety Authority Panel on Contaminants in the Food Chain. 2021. Scientific report on the chronic dietary exposure to inorganic arsenic. EFSAshort forEuropean Food Safety Authority Journal. 19(1), 6380. DOI: 10.2903/j.efsa.2021.6380
EFSAshort forEuropean Food Safety Authority (2023): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2023. Re-evaluation of the existing health-based guidance values for copper and exposure assessment from all sources. EFSAshort forEuropean Food Safety Authority Journal. 21(1), 7728. DOI: 10.2903/j.efsa.2023.7728
EFSAshort forEuropean Food Safety Authority (2024, a): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2024. Update of the risk assessment of inorganic arsenic in food. EFSAshort forEuropean Food Safety Authority Journal.22, e8488. DOI: 10.2903/j.efsa.2024.8488
EFSAshort forEuropean Food Safety Authority (2024, b): EFSAshort forEuropean Food Safety Authority Scientific Committee. 2024. Overview on Tolerable Upper Intake Levels as derived by the Scientific Committee on Food (SCF) and the EFSAshort forEuropean Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies (NDA), European Food Safety Authority, 2024. www.efsa.europa.eu/sites/default/files/2024-05/ul-summary-report.pdf
EFSAshort forEuropean Food Safety Authority and ECHAshort forEuropean Chemicals Agency (2021): European Food Safety Authority (EFSAshort forEuropean Food Safety Authority) and European Chemicals Agency (ECHAshort forEuropean Chemicals Agency). 2021. Memorandum of Understanding between the European Chemicals Agency (ECHAshort forEuropean Chemicals Agency) and the European Food Safety Authority (EFSAshort forEuropean Food Safety Authority) Comparison of the evaluations performed on silver compounds used as biocidal active substances in food contact materials (FCM) by EFSAshort forEuropean Food Safety Authority and ECHAshort forEuropean Chemicals Agency: www.efsa.europa.eu/sites/default/files/2021-02/joint-efsa-echa-comparison-evaluations-perfomed-silver-compounds-biocidal-active-substances-fcm.pdf
EFSAshort forEuropean Food Safety Authority CONTAM (2010): EFSAshort forEuropean Food Safety Authority Panel on Contaminants in the Food Chain (EFSAshort forEuropean Food Safety Authority CONTAM). 2010. Scientific Opinion of the EFSAshort forEuropean Food Safety Authority Panel on Contaminants in the Food Chain (CONTAM) on Lead in Food. EFSAshort forEuropean Food Safety Authority Journal. 8 (4), 1570. DOI: 10.2903/j.efsa.2010.1570
EFSAshort forEuropean Food Safety Authority CONTAM (2014): EFSAshort forEuropean Food Safety Authority Panel on Contaminants in the Food Chain (EFSAshort forEuropean Food Safety Authority CONTAM). 2014. Scientific Opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSAshort forEuropean Food Safety Authority Journal. 12, 3595. DOI: 10.2903/j.efsa.2014.3595
EFSAshort forEuropean Food Safety Authority CONTAM (2020): EFSAshort forEuropean Food Safety Authority Panel on Contaminants in the Food Chain (EFSAshort forEuropean Food Safety Authority CONTAM). 2020. Scientific Opinion on the update of the risk assessment of nickel in food and drinking water. EFSAshort forEuropean Food Safety Authority Journal. 18(11), 6268. DOI: 10.2903/j.efsa.2020.6268
EFSAshort forEuropean Food Safety Authority NDA (2023): EFSAshort forEuropean Food Safety Authority Panel on Nutrition, Novel Foods and Food Allergens (NDA). 2023. Scientific opinion on the tolerable upper intake level for selenium. EFSAshort forEuropean Food Safety Authority Journal. 21(1), 7704. DOI: 10.2903/j.efsa.2023.7704
EFSAshort forEuropean Food Safety Authority NDA (2023): EFSAshort forEuropean Food Safety Authority Panel on Nutrition, Novel Foods and Food Allergens (NDA). 2023. Scientific opinion on the tolerable upper intake level for manganese. EFSAshort forEuropean Food Safety Authority Journal. 21(11), e8413. DOI: 10.2903/j.efsa.2023.8413
EFSAshort forEuropean Food Safety Authority NDA (2024): EFSAshort forEuropean Food Safety Authority Panel on Nutrition, Novel Foods and Food Allergens (NDA). 2024. Scientific opinion on the tolerable upper intake level for iron. EFSAshort forEuropean Food Safety Authority Journal. 22(6). DOI: 10.2903/j.efsa.2024.8819
EPA (2008): Environmental Protection Agency (EPA). 2008. Provisional Peer Reviewed Toxicity Values for Lithium (CASRN 7439-93-2). Superfund Health Risk Technical Support Centre National Centre for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency EPA/690/R-08/016F
EPA (2009): Environmental Protection Agency (EPA). 2009. Toxicological Review of Thallium and compounds (CAS No. 7440-28-0). In Support of Summary Information on the Integrated Risk Information System (IRIS). U.S. Environmental Protection Agency. EPA/635/R-08/001F.
EPA (2012): Environmental Protection Agency (EPA). 2012. Provisional Peer-Reviewed Toxicity Values for Thallium and Compounds. U.S. Environmental Protection Agency. EPA/690/R-12/028F
EU-FORA (2022): European Food Safety Authority (EFSAshort forEuropean Food Safety Authority). 2022. Risk assessment of rare earth elements, antimony, barium, boron, lithium, tellurium, thallium and vanadium in teas. EFSAshort forEuropean Food Safety Authority Journal. 20(S1):e200410, 12 pp. DOI: 10.2903/j.efsa.2022.e200410
EVM (2003): Expert Group on Vitamins and Minerals (EVM). 2003. Safe Upper Levels for Vitamins and Minerals. Food Standards Agency. ISBN 1-904026-11-7. cot.food.gov.uk/sites/default/files/cot/vitmin2003.pdf
Fairweather-Tait et al.short foret alii (lat. "and others") (2011): Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Hesketh J.E., Hurst R. 2011. Selenium in human health and disease. Antioxidants and Redox Signalling. 14, 1337–1383. DOI: 10.1089/ars.2010.3275
Fawcett et al.short foret alii (lat. "and others") (1997): Fawcett J.Pshort forphosphorus., Farquhar S.J., Thou T., Shand B.I. 1997. Oral vanadyl sulphate does not affect blood cells, viscosity or biochemistry in humans. Pharmacology & Toxicology. 80, 202-206. DOI: 10.1111/j.1600-0773.1997.tb00397.x
Filippini et al.short foret alii (lat. "and others") (2020): Filippini T., Tancredi S., Malagoli C., Malavolti M., Bargellini A., Vescovi L., Nicolini F., Vinceti M. 2020. Dietary Estimated Intake of Trace Elements: Risk Assessment in an Italian Population. Exposure and Health. 12, 641–655. DOI: 10.1007/s12403-019-00324-w
FSA (2014): Food Standards Agency (FSA). 2014. Total diet study: Metals and other elements - Data table, 2014. www.food.gov.uk/research/chemical-hazards-in-food-and-feed/total-diet-study-metals-and-other-elementsdata-filee: www.food.gov.uk/sites/default/files/media/document/metals-exposure-data.xlsx
Fungwe et al.short foret alii (lat. "and others") (1990): Fungwe T.V., Buddingh F., Demick D.S., Lox C.D., Yang M.T., Yang S.Pshort forphosphorus. 1990. The role of dietary molybdenum on oestrus activity, fertility, reproduction and molybdenum and copper enzyme activities of female rats. Nutrition Research. 10, 515-524. DOI: 10.1016/S0271-5317(05)80061-2
Greene et al.short foret alii (lat. "and others") (2025): Greene C.W., Valcke M., Soshilov A.A., Levallois Pshort forphosphorus., Goeden H.M. 2025. A review of common approaches to determining allocation factors and relative source contribution factors for drinking water contaminants: caveats and areas for improvement. Regulatory Toxicology and Pharmacology. 162, 105886. DOI: 10.1016/j.yrtph.2025.105886
Health Canada (2005): Health Canada. 2005. Canadian total diet study. Ottawa, Ontario: Health Canada. www.hcsc.gc.ca/food-aliment/cs-ipc/fr-ra/e_tds.html
Hosokawa et al.short foret alii (lat. "and others") (1994): Hosokawa S., Yoshida Oshort foroxygen. 1994. Clinical studies on molybdenum in patients requiring long-term haemodialysis. ASAIO Journal. 40(3), M445-449
IARC (2012): International Agency for Research on Cancer (IARC). 2012. IARC monograph on the evaluation of carcinogenic risks to humans - arsenic, metals, fibres, and dusts. IARC Press, Lyon, France. 100C, 41-94. ISBN: 978 92 832 1320 8
ICH (2022): International Council for Harmonisation (ICH). 2022. ICH guideline Q3D (R2) on elemental impurities. www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use-ich-q3d-elemental-impurities-step-5-revision-2_en.pdf
Iordache et al.short foret alii (lat. "and others") (2024): Iordache A.M., Voica C., Roba C., Nechita C. 2024. Lithium Content and Its Nutritional Beneficence, Dietary Intake, and Impact on Human Health in Edibles from the Romanian Market. Foods. 13(4):592. DOI: 10.3390/foods13040592
JECFA (2012): Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2012. Safety evaluation of certain food additives and contaminants. World Health Organisation. ISBN: 978 92 4 166065 5. apps.who.int/iris/handle/10665/44813
Kern et al.short foret alii (lat. "and others") (2010): Kern C.H., Stanwood G.D., Smith D.R. 2010. Pre-weaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 64(5), 363-78. DOI: 10.1002/syn.20736
Kohlbaum et al.short foret alii (lat. "and others") (2019): Kolbaum A.E., Berg K., Müller F., Kappenstein Oshort foroxygen., and Lindtner Oshort foroxygen. 2019. Dietary Exposure to Elements from the first German Pilot Total Diet Study (TDS). Food Additives & Contaminants. Part A. 36 (12), 1822-1836. DOI: 10.1080/19440049.2019.1668967
Kravchenko et al.short foret alii (lat. "and others") (2014): Kravchenko J., Darrah T.H., Miller R.K., Lyerly H.K., Vengosh A. 2014. A review of the health impacts of barium from natural and anthropogenic exposure. Environmental Geochemistry and Health. 36, 797–814. DOI: 10.1007/s10653-014-9622-7
Laupheimer et al.short foret alii (lat. "and others") (2025): Laupheimer C.E., Kolianchuk Y., FitzGerald R.E., Wilks M.F., Jaksch A. 2025. Toxicological evaluation of vanadium and derivation of a parenteral tolerable intake value for medical devices. Regulatory Toxicology and Pharmacology. 156, 105732. DOI: 10.1016/j.yrtph.2024.105732
Lippman et al.short foret alii (lat. "and others") (2009): Lippman S.M., Klein E.A., Goodman Pshort forphosphorus.J., Lucia M.S., Thompson I.M., Ford L.G., Parnes H.L., Minasian L.M., Gaziano J.M., Hartline J.A., Parsons J.K., Bearden J.D., Crawford E.D., Goodman G.E., Claudio J., Winquist E., Cook E.D., Karp D.D., Walther Pshort forphosphorus., Lieber M.M., Kristal A.R., Darke A.K., Arnold K.B., Ganz Pshort forphosphorus.A., Santella R.M., Albanes D., Taylor Pshort forphosphorus.R., Probstfield J.L., Jagpal T.J., Crowley J.J., Meyskens F.L., Baker L.H., Coltman C.A. 2009. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). The Journal of the American Medical Association. 301, 39–51. DOI: 10.1001/jama.2008.864
Lynch et al.short foret alii (lat. "and others") (1999): Lynch B.S., Capen C.C., Nestmann E.R., Veenstra G., Deyo J.A. 1999. Review of subchronic/chronic toxicity of antimony potassium tartrate. Regulatory Toxicology and Pharmacology. 30 (1), 9-17. DOI: 10.1006/rtph.1999.1312
McKnight et al.short foret alii (lat. "and others") (2012): McKnight R.F., Adida M., Budge K., Stockton S., Goodwin G.M., Geddes J. R. 2012. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 379, 721–749. DOI: 10.1016/S0140
MRIshort forMax Rubner Institute (2022): Max Rubner Institute (MRIshort forMax Rubner Institute). 2022. National Nutrition Survey (LODshort forLimit of detection) II, Results Reports 1 and 2. Max Rubner Institute, Federal Research Institute for Nutrition and Food. www.mri.bund.de/de/institute/ernaehrungsverhalten/forschungsprojekte/nvsii/erg-verzehr-naehrstoffe/ and www.mri.bund.de/fileadmin/MRI/Institute/EV/NVSII_Abschlussbericht_Teil_2.pdf
NTP (2023): National Toxicology Program (NTP). 2023. NTP Technical Report on the Toxicity Studies of Sodium Metavanadate (CASRN 13718-26-8) and Vanadyl Sulfate (CASRN 27774-13-6) Administered in Drinking Water to Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and B6C3F1/N Mice: Toxicity Report 106. Research Triangle Park (NC): National Toxicology Programme. DOI: 10.22427/NTP-TOX-106
Poon et al.short foret alii (lat. "and others") (1988): Poon R., Chu I., Lecavalier Pshort forphosphorus., Valli V.E., Foster W., Gupta S., Thomas B. 1998. Effects of antimony on rats following 90-day exposure via drinking water. Food and Chemical Toxicology. 36 (1), 21-35. www.ncbi.nlm.nih.gov/pubmed/9487361
RAC (2020): Committee for Risk Assessment (RAC). 2020. Opinion proposing harmonised classification and labelling at EU level of divanadium pentaoxide; vanadium pentoxide. European Chemicals Agency. Report: CLH-Oshort foroxygen-0000006927-60-01/F. echa.europa.eu/documents/10162/6c9565dd-6350-5498-4d9d-b239ddbc88c8
Rahman et al.short foret alii (lat. "and others") (2009): Rahman A., Vahter M., Smith A.H., Nermell B., Yunus M., El Arifeen S., Persson L.Å., and Ekström E.C. 2009: Arsenic Exposure During Pregnancy and Size at Birth: A Prospective Cohort Study in Bangladesh. American Journal of Epidemiology. 169 (3), 304-312. DOI: 10.1093/aje/kwn332
RIVM (2001): National Institute for Public Health and the Environment (RIVM). 2001. Re-evaluation of human-toxicological maximum permissible risk levels. RIVM Report 711701 025. www.rivm.nl/bibliotheek/rapporten/711701025.pdf
RIVM (2009): National Institute for Public Health and the Environment (RIVM). 2009. Re-evaluation of some human-toxicological maximum permissible risk levels previously evaluated in the period 1991-2001, RIVM Report 711701092/2009. www.rivm.nl/bibliotheek/rapporten/711701092.pdf
SCF (2000): Scientific Committee on Food (SCF). 2000. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Molybdenum, European Commission. ec.europa.eu/food/fs/sc/scf/out80h_en.pdf
SCF (2003): Scientific Committee on Food (SCF). 2003. Opinion of the Scientific Committee on Food on the tolerable upper intake level of Zinc. European Commission. ec.europa.eu/food/fs/sc/scf/out177_en.pdf
SCHER (2012): Scientific Committee on Health and Environmental Risks (SCHER). 2012. Assessment of the tolerable daily intake of barium. European Union. DOI: 10.2772/49651
Sherlock et al.short foret alii (lat. "and others") (1986): Sherlock J.C., Smart G.A. 1986. Thallium in foods and the diet. Food Additives & Contaminants. 3(4):363-70. DOI: 10.1080/02652038609373603
UBAshort forGerman Environment Agency (2011): Federal Environment Agency (UBAshort forGerman Environment Agency). 2011. Substance Monograph Thallium – Reference and Human Biomonitoring (HBM) Values for Thallium in Urine. Bundesgesundheitsblatt. 54, 516-24. DOI: 10.1007/s00103-011-1252-y
Valcke et al.short foret alii (lat. "and others") (2018): Valcke M., Bourgault M.H., Haddad S., Bouchard M., Gauvin D., Levallois Pshort forphosphorus. 2018. Deriving a drinking water guideline for a non-carcinogenic contaminant: the case of manganese. International Journal of Environmental Research and Public Health. 15(6)1293. DOI: 10.3390/ijerph15061293
Vyskocil et al.short foret alii (lat. "and others") (1999): Vyskocil A., Viau C. 1999. Assessment of molybdenum toxicity in humans. Journal of Applied Toxicology. 19, 185-192. DOI: 10.1002/(sici)1099-1263(199905/06)19:3<185::aid-jat555>3.0.co;2-z
WHO (1996): World Health Organisation (WHO) & International Programme for Chemical Safety. 1996. Thallium. World Health Organisation. iris.who.int/handle/10665/37751. Report: www.inchem.org/documents/ehc/ehc/ehc182.htm
WHO (2003): World Health Organisation (WHO). 2003. Antimony in Drinking-water ¬¬¬¬– Background document for development of WHO Guidelines for Drinking-water Quality. cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/antimony.pdf
WHO (2017): World Health Organisation (WHO). 2022. Guidelines for drinking-water quality: Fourth edition Incorporating the first addendum. ISBN 978-92-4-154995-0. iris.who.int/bitstream/handle/10665/254637/9789241549950-eng.pdf
WHO (2022): World Health Organization (WHO). 2022. Guidelines for drinking-water quality: Fourth edition Incorporating the first and second addenda. ISBN: 978-92-4-004506-4. iris.who.int/bitstream/handle/10665/254637/9789241549950-eng.pdf
Ysart et al.short foret alii (lat. "and others") (1999): Ysart G., Miller Pshort forphosphorus., Crews H., Robb Pshort forphosphorus., Baxter M., L’Argy C.D., Lofthouse S., Sargent C., Harrison N. 1999. Dietary exposure estimates of 30 elements from the UK Total Diet Study. Food Additives & Contaminants, 16(9), 391–403. DOI: 10.1080/026520399283876