79
the BfR did not identify directly genotoxic or mutagenic
properties of nano-titanium dioxide, if tested at exposure
levels which could be expected under normal conditions.
Finally, the BfR also took part in the inter-laboratory test
involving the intestinal cells which were identified as sen-
sitive cell systems for
in vitro
genotoxicity testing during
the first phase of the project.
Future work will focus on the establishment of appro-
priate methods which allow quantifying the intracellular
amount/dose of nanoparticles in cells which can come
into contact with nanomaterials, after a “real-life” expo-
sure treatment. With respect to this, the BfR participates
in a Franco-German follow-up project.
Application guideline for tests for
skin irritants and corrosives
The determination of skin-irritant or skin-corrosive prop-
erties is legally required for the toxicological assessment
of cosmetic ingredients and industrial chemicals. The
main method of testing for these properties to date has
involved animal experiments on rabbits. As the new Cos-
metics Regulation contains a European marketing ban
from July 2013 on cosmetics containing ingredients that
have been tested on animals, this testing method can no
longer be used, at least in the case of these products.
In the last ten years, artificial human skin models have
been developed which closely imitate the texture and
properties of human skin. These so-called Reconstructed
Human Epidermis (RHE) models are based on the
in vitro
differentiation of human skin cells into three-dimensional
tissue structures. On the basis of these skin models, the
BfR worked with international partners to develop and val-
idate tests for corrosive and irritant properties of various
substances and products. These tests are recognised for
official purposes around the world as OECD test guide-
line 431 (
in vitro
skin corrosion) and OECD test guideline
439 (
in vitro
skin irritation). By combining these two test
methods, harmful tests on rabbits which were previously
used can be replaced completely.
When investigating the genotoxicity of nanomaterials in
vitro, the BfR works with three-dimensionally reconstructed
human skin models that are grown in so-called 6-well
plates during the test.
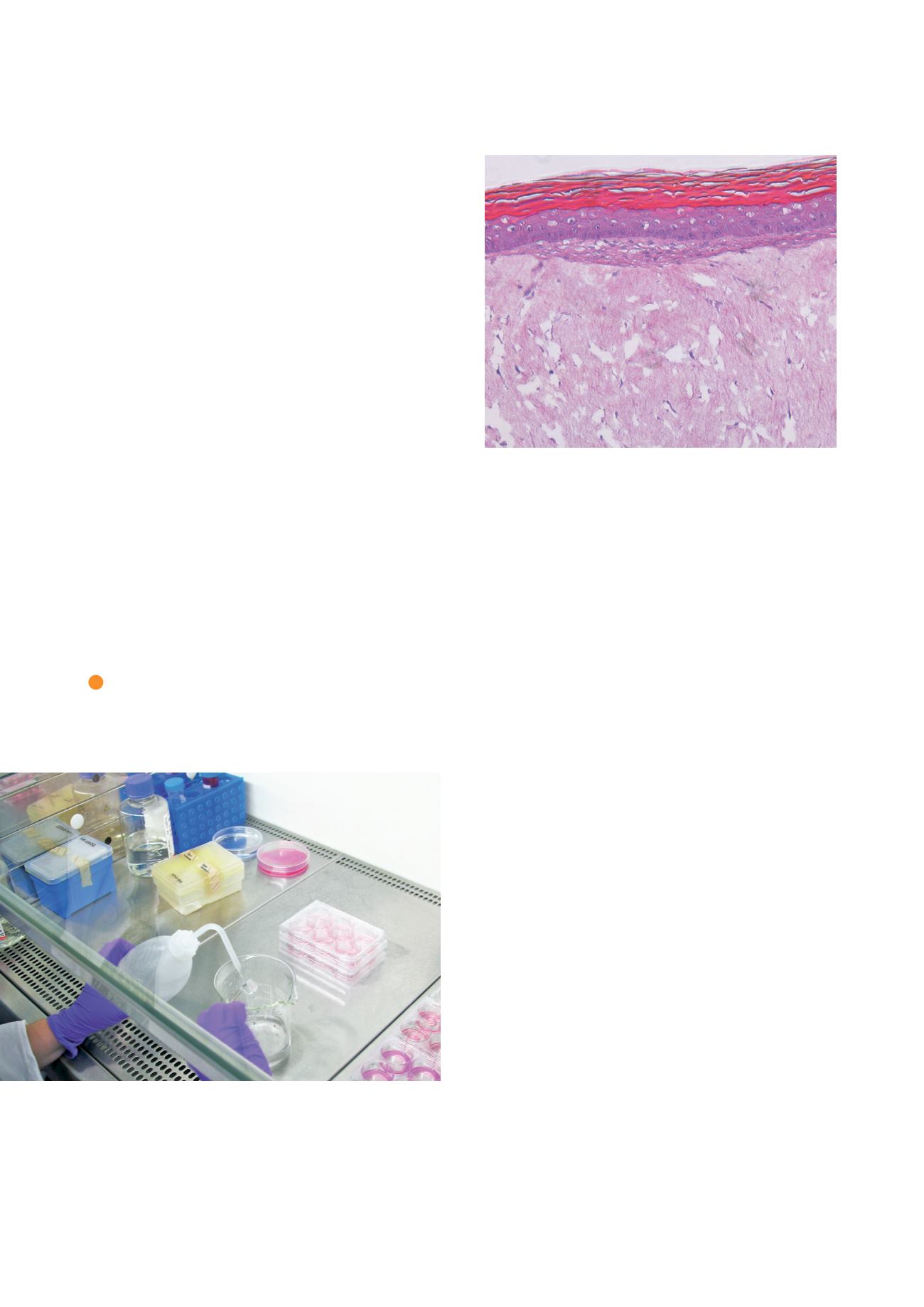
Histology of skin model shows epidermis consisting of
stratum corneum and basal, spinous and granular keratino-
cytes (from top to bottom). Dermis contains numerous
viable fibroblasts.
Alternatives to Animal Experiments


